Search all Frequently Asked Questions
Annual continuing review is generally required for protocols approved by the fully convened IRB committee. An annual continuing review may also be necessary for the following types of protocols:
- Projects involving Native American or Indigenous Populations;
- Principal Investigator has received serious or continuing non-compliance determinations in the past two years;
- Projects that involve deception but do not receive the subject’s prior authorization to be deceived before engaging in the deception;
- A Conflict of Interest Management Plan exists;
- FDA-regulated research eligible for Expedited review under Expedite Category 1 on approved drugs or devices;
- Projects deemed Expedited Category 9; or
- As determined by the IRB on a project basis depending on the risks in the research project.
As a courtesy, eIRB notifications are sent to investigators several weeks before the approval end date for protocols needing a continuing review. A continuing review submission must be submitted in eIRB at least 4-6 weeks before the approval end date. If the protocol is not granted continuing approval before expiration, all research activities must pause until reapproval.
Please review HSPP Guidance, Continuing Review of Human Research for more information.
Because the stipend is less than $5,000 and from a U.S. institution, this does not need to be disclosed for conflict of interest review. This may, however, need to be disclosed for conflict of commitment review if it is an Outside Commitment, which can include fee-for-service activity and Research.
The Outside Commitment Decision Tree on our Disclosure Requirements webpage are available to assist individuals in determining what needs to be disclosed. Individuals can also contact OROI at coi@arizona.edu.
COI: Income received from the University of Arizona is exempt from the COI disclosure requirements
COC: Please work with your college/unit leadership to determine if they consider this to be an Outside Commitment. Pursuant to policy, Outside Commitments (1) are professional and other activities that are related to a University Employee’s professional expertise, outside of their University duties and responsibilities; (2) are for the benefit of an external entity or individual and are not covered by a fully executed written agreement between the University and the external entity; and (3) require a time commitment. Here, the teaching commitment is not for the benefit of an external entity or individual. With that said, an individual’s supervisor/department/college could require submission of a COC form for review and approval to ensure the individual’s institutional duties and responsibilities are properly covered if they desired to do so.
Outside Commitments require prior approval.
RII also believes that it was important to get feedback from Faculty Senate and other stakeholders. As such, the policy underwent multiple stakeholder reviews, including review by Faculty Senate and APPC. It was presented to the full Faculty Senate at its December 2, 2019, senate meeting and circulated to the Faculty Senate for review prior to implementation on an interim basis in May 2021. It was also discussed at a January 9, 2020 APPC meeting in which Taren Ellis Langford was present. (Please know that all suggestions and edits from APPC were incorporated into the final policy.)
Here is the full list of stakeholders who were provided a copy of the draft policy and invited to participate in the review, feedback and comment period:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
If you will be participating in research under the auspices of the University, you are required to complete the Required COI Disclosure Training through Edge Learning and submit a disclosure via via eDisclosure. These requirements apply to anyone who is an “Investigator” on a research project at the University (whether or not externally funded). “Investigator” is a defined term in the Conflicts of Interest and Commitment policy, and generally means “any person who shares the responsibility for the design, conduct, or reporting of Research” and may include students, postdocs and trainees.
More details can be found on the Conflict of Interest Requirements for Students, Postdocs and Trainees webpage.
Yes, since UArizona receives federal funding, all University Employees are expected to follow federal regulations as embodied in the Conflicts of Interest & Commitment Policy.
If you are an Investigator, this activity should be disclosed for conflict of interest review if it meets the definition of a Significant Personnel Interest. Significant Personal Interests are any managerial, professional, or Fiduciary Position you (or a Family Member) hold in any outside entity, whether or not you or your family is compensated. This can include officer, director, and board positions.
Fiduciary Position means one's legal and/or ethical obligation to act in the best interests (e.g., the financial and/or operating success) of another person or entity, regardless of whether such role is compensated. Examples of Fiduciary Positions include but are not limited to membership on a board of directors or board of advisors, or a management role in an entity (e.g., as a corporate officer, LLC member, general partner, and governing board member of a professional association).
If this activity meets the definition of an Outside Commitment, it should be disclosed for conflict of commitment review. The Outside Commitment Decision Tree on our Disclosure Requirements webpage may be of assistance in making this determination. Individuals can also contact OROI at coi@arizona.edu.
Please work with your college to determine if this is outside of your institutional duties and responsibilities. If this activity meets the definition of an Outside Commitment, it should be disclosed for conflict of commitment review. The Outside Commitment Decision Tree on our Disclosure Requirements webpage may be of assistance in making this determination.
If you are an Investigator, receipt of remuneration (includes stipends and honorariums) in the amount of $5,000 or more will make this a Significant Financial Interest that needs to be disclosed.
If you are an Investigator and receive remuneration (includes stipends and honorariums) of any amount from a foreign entity, this must be disclosed as a Foreign Interest for conflict of interest review.
There is not enough information to determine whether this should be disclosed for conflict of interest review. (e.g., Is it a UArizona grant? Are you funded by a PHS agency or the Dept of Energy?) Please contact OROI at coi@arizona.edu or visit our office hours (1st & 3rd Thursday, 2 pm – 3 pm; Connect via Zoom) for assistance.
If this activity meets the definition of an Outside Commitment, it should be disclosed for conflict of commitment review. The Outside Commitment Decision Tree on our Disclosure Requirements webpage may be of assistance in making this determination.
If you are an Investigator, this activity should be disclosed for conflict of interest review if it meets the definition of a Significant Personnel Interest. Significant Personal Interests are any managerial, professional, or Fiduciary Position you (or a Family Member) hold in any outside entity, whether or not you or your family is compensated. This can include officer, director, and board positions.
Fiduciary Position means one's legal and/or ethical obligation to act in the best interests (e.g., the financial and/or operating success) of another person or entity, regardless of whether such role is compensated. Examples of Fiduciary Positions include but are not limited to membership on a board of directors or board of advisors, or a management role in an entity (e.g., as a corporate officer, LLC member, general partner, and governing board member of a professional association).
If this activity meets the definition of an Outside Commitment, it should be disclosed for conflict of commitment review. The Outside Commitment Decision Tree on our Disclosure Requirements webpage may be of assistance in making this determination. Individuals can also contact OROI at coi@arizona.edu.
Investigators, as defined in the Conflicts of Interest & Commitment policy, are required to submit a Research Certification for each Research Project, both non-sponsored and sponsored. OROI relies on the PI to make this determination. “Who is an Investigator?” can be used to help determine if you are an Investigator.
For IRB protocols, the Human Subjects Protection Program has guidelines for who needs to submit a Research Certification - Investigator Roles & COI Disclosures in eIRB. OROI happy to assist you in contacting them or you can reach out to them via email - vpr-irb@email.arizona.edu.
An up-to-date COI disclosure (either an Annual Disclosure or Research Certification submitted within the last 364 days) is required at the time of proposal to a federal funding agency.
Additionally, Federal regulations prohibit expenditures on Awards until after the COI review process is complete. Our office desires to see all funded research go forward without delay.
Therefore, to avoid Award Holds, you are asked to submit Research Certifications early. Generally, Research Certifications are available in eDisclosure 60 days prior to the project start date listed in the Institutional Proposal. (For certain clinical trials, it may be fewer than 60 days) eDisclosure will send a notification to the Investigator as part of the UAccess Research/Sponsored Projects integration. If at any time you have been informed that any given project was not funded you would then advise sponsor@arizona.edu as soon as possible that the specific project will not be funded and no action is required for the Research Certification.
Please work with your college to determine if this is outside of your institutional duties and responsibilities. If this activity meets the definition of an Outside Commitment, it should be disclosed for conflict of commitment review. The Outside Commitment Decision Tree on our Disclosure Requirements webpage may be of assistance in making this determination.
If you are an Investigator, receipt of remuneration (includes stipends and honorariums) in the amount of $5,000 or more will make this a Significant Financial Interest that needs to be disclosed.
If the Outside Commitment is not approved, the individual cannot engage in the Outside Commitment.
OROI is available to work with college/department approvers to discuss concerns and develop a COC management plan. More details about the COC review process are available on our COC & COI Review Processes webpage.
This depends on your UA role (whether an Employee, Administrator and/or Investigator – An complete overview of UA roles and Disclosure requirements can be found here)
- If you are an Investigator, receiving remuneration/income in any amount from Intellectual Property rights, such as patents or copyrights will make this a Significant Financial Interest that needs to be disclosed via eDisclosure. Additionally,
- If you are a UA employee whose FTE is 0.5 or greater, the definition of Outside Employment and Outside Commitment is included below for your review. The Outside Commitment Decision Tree may be of assistance when making the determination as to whether an activity requires disclosure. Additionally, the Disclosure Table resources provide an overview of the disclosure requirements.
Outside Employment refers to any employment relationship outside of the University requiring a time commitment.
Outside Commitments: (1) are professional and other activities that are related to a University Employee’s professional expertise, outside of their University duties and responsibilities; (2) are for the benefit of an external entity or individual and are not covered by a fully executed written agreement between the University and the external entity; and (3) require a time commitment. Outside Commitments include Outside Employment, independent contracts for consulting services, private consulting groups comprised of University Employees, volunteer/pro bono work, appointments at postsecondary educational institutions, and foreign components, as that term may be updated by the University’s Office for Responsible Outside Interests.
Also, please refer to the University’s TLA-100 The Intellectual Property Policy in case applicable.
Both. Outside Commitments must be disclosed in eDisclosure, at which time OROI will initiate the review process.
More details about the COC review process are available on our COC & COI Review Processes webpage.
Generally speaking, this often falls within one’s institutional duties and responsibilities. If this is outside of an individual’s institutional duties and responsibilities and meets the definition of an Outside Commitment, it will need to be disclosed for conflict of commitment review.
This activity falls under the following exemption for conflict of interest disclosure: Income from seminars, lectures, teaching engagements, or service on advisory committees or review panels sponsored by (i) a government agency (federal, state, or local); or (ii) an institution of higher education as defined at 20 USC § 1001(a); or (iii) an academic teaching hospital, medical center, or research institute that is affiliated with an institution of higher education.
If this activity meets the definition of an Outside Commitment, it should be disclosed for conflict of commitment review. The Outside Commitment Decision Tree on our Disclosure Requirements webpage may be of assistance in making this determination.
If you are an Investigator, receipt of remuneration (includes stipends and honorariums) in the amount of $5,000 or more will make this a Significant Financial Interest that needs to be disclosed.
Disclosures must be submitted in eDisclosure. If you experience any issues in eDisclosure, please contact OROI at coi@arizona.edu.
University Employees are asked to disclose their Outside Interests (Significant Financial Interests, Significant Personal Interests, Foreign Interests), Outside Commitments and Substantial Interests so that determinations of what is and is not a conflict can made through OROI. The review processes are available on our COC & COI Review Processes webpage.
The Disclosure Tables and Outside Commitment Decision Tree on our Disclosure Requirements webpage are available to assist individuals in determining what needs to be disclosed. Individuals can also contact OROI at coi@arizona.edu.
Financial Conflict of Interest means an Outside Interest is Related to, or can be perceived to be Related to, an individual’s institutional responsibilities. Relatedness is a defined term that means it may reasonably appear that decisions made by the Investigator in the performance of his/her institutional responsibilities could directly and significantly affect the value of his/her Significant Financial Interests or be in conflict with Significant Personal Interests or Foreign Interests. More information can be found here: Relatedness.
Outside Commitments require prior approval. In an ideal world, the COC form would be submitted in eDisclosure 4 weeks prior to the start date to ensure college and department reviewers have an opportunity to review the form, resolve concerns and/or implement a management plan. Realizing that submission 4 weeks prior to the start date is not always possible, we ask that individuals email us to flag a fast-approaching start date so that we can work with the approvers to ensure all questions are answered, etc.
A foreign entity is:
- A public or private organization located in a country other than the United States and its territories that is subject to the laws of the country in which it is located, irrespective of the citizenship of project staff or place of performance; or
- A private nongovernmental organization located in a country other than the United States that solicits and receives cash contributions from the general public; or
- A charitable organization located in a country other than the United States that is nonprofit and tax exempt under the laws of its country of domicile and operation, and is not a university, college, accredited degree granting institution of education, private foundation, hospital, organization engaged exclusively in research or scientific activities, church, synagogue, mosque or other similar entities organized primarily for religious purposes; or
- An organization located in a country other than the United States not recognized as a Foreign Public Entity. A Foreign Public Entity is (1) A foreign government or foreign governmental entity; (2) A public international organization, which is an organization entitled to enjoy privileges, exemptions, and immunities as an international organization under the International Organizations Immunities Act (22 U.S.C. 288f); (3) An entity owned (in whole or in part) or controlled by a foreign government; or (4) Any other entity consisting wholly or partially of one or more foreign governments or foreign governmental entities.
Please work with your college to determine what is and is not considered to be part of your UArizona duties and responsibilities. The following disclosure scenarios may be helpful:
- An Investigator receives personal compensation or an honorarium of $5,000 or more for editing journal articles. In this instance, the editing work must be disclosed as a Significant Financial Interest for conflict of interest (COI) review even though it is part of the individual’s professional service requirement. The Investigator would not need to submit a COC form for approval if the activity is part of their professional service.
- An Investigator serves on the Scientific Advisory Board for a professional society but does not receive any remuneration. The Investigator must disclose this board membership as a Significant Personal Interest for conflict of interest (COI) review. The Investigator would not need to submit a COC form for approval if the activity is part of their professional service.
- An Investigator receives an honorarium of $300 from a foreign funding agency to review research proposals. In this instance, the Investigator must disclose the review work as a Foreign Interest for conflict of interest (COI) even though the remuneration is less than $5,000 and the work may be part of the individual’s professional service. The Investigator would not need to submit a COC form for approval if the activity is part of their professional service.
Research and Research Project mean any organized program of scientific inquiry that involves a systematic investigation, study, or experiment designed to develop or contribute to generalizable knowledge that is performed at or under the auspices of the University. Research includes non-sponsored research, research fellowship and training programs, and research-related activities in undergraduate, graduate, and postdoctoral education. It also includes some educational activities that are supported by a research sponsor.
Federal funding agencies indicate that Research can be thought of as:
- "a process to discover new knowledge,"
- "a scientific study of nature that sometimes includes processes involved in health and disease," and/or
- "creative and systematic work undertaken in order to increase the stock of knowledge—including knowledge of humankind, culture and society—and to devise new applications of available knowledge."
Federal funding agencies further indicate that one can consider whether the project includes:
- "a systematic, intensive study directed toward greater knowledge or understanding of the subject being studied, or
- a systematic study directed specifically toward applying new knowledge to meet a recognized need, or
- a systematic application of knowledge to produce useful materials, devices, and systems or methods, or
- development [which] may include designing, developing, and improving prototypes and processes to meet specific requirements."
Overlap between an Outside Interest and a Research Project occurs when there is Relatedness.
“Relatedness” is the condition in which it may reasonably appear that decisions made by the Investigator in the performance of his/her institutional responsibilities could directly and significantly affect the value of his/her Significant Financial Interests or be in conflict with Significant Personal Interests or Foreign Interests.
Relatedness includes situations in which an Investigator’s Outside Interests would reasonably appear to affect, or to be affected by, the individual’s Research or other institutional responsibilities, as well as situations in which the Outside Interest involves an entity whose financial interests would reasonably appear to affect, or be affected by, the Investigator’s Conduct of Research or other institutional responsibilities.
Relatedness is not a judgment on whether the Investigator would deliberately make choices in the Conduct of Research or the performance of his/her Institutional Responsibilities based on considerations related to his/her Significant Financial Interest, Significant Personal Interest or Foreign Interest. Rather, “Relatedness” refers to the condition in which it may reasonably appear that choices made in the Conduct of Research or other performance of the individual’s institutional responsibilities could be directly and significantly influenced by the existence of Outside Interests.
If you are an Investigator, this is a Foreign Interest that must be disclosed for conflict of interest review. Foreign Interests are:
- Participation in a foreign talent or similar-type program
- All resources and other support, both domestic and foreign, for ongoing research projects, including those conducted at a different institution
- In-kind contributions from domestic and foreign institutions or governments that support your research activities
- Any payment, reimbursement, travel support or other compensation, of any amount, that you personally receive, or will personally receive, from a foreign entity
(If this was a U.S. institution, it would need to be disclosed if you are funded by a PHS agency or the Dept of Energy, even if the value is less than $5,000.)
If your UArizona FTE is 0.50 or greater and this activity meets the definition of an Outside Commitment, it should be disclosed for conflict of commitment review. The Outside Commitment Decision Tree on our Disclosure Requirementswebpage may be of assistance in making this determination. Individuals can also contact OROI at coi@arizona.edu.
Also, please see: Guidance for Consulting or Employment at Other Postsecondary Institutions.
“It is permissible for members of the faculty on sabbatical leave to supplement their compensation from the university to cover such special expenses resulting from the approved sabbatical leave program, through fellowships, scholarships, employment, or grants-in-aid. Such special expenses referred to might include such items as travel, secretarial assistants, tuition, research, and publication. Additional compensation expected is to be fully explained on the application form and approved before the leave is granted. Should opportunities for supplemental compensation develop after the sabbatical leave has begun or after the application form has been submitted and approved, such opportunities must be cleared with the university at the earliest opportunity.” See ABOR 6-207(F).
In the past, University Employees were asked to disclose Outside Commitments in the COC database and Outside Interests in the COI database.
The Conflicts of Interest & Commitment Policy incorporated and replaced the following policies:
1. Conflict of Commitment Policy
2. Conflict of Interest (UHAP) Policy
3. Conflict of Interest in Purchasing Policy
4. Individual Conflict of Interest in Research Policy
5. Institutional Conflict of Interest Policy
Now, eDisclosure serves as a single platform to meet all policy disclosure requirements.
If you are an Investigator and receive a travel reimbursement/sponsorship and/or stipend of any amount from a foreign entity, this must be disclosed as a Foreign Interest for conflict of interest review.
If you are an Investigator and receive a stipend of $5,000 or more, this must be disclosed as a Significant Financial Interest for conflict of interest review.
If you are an Investigator who has funding from a PHS agency or the Department of Energy and receive a travel sponsorship or reimbursement of any amount, this must be disclosed as a Significant Financial Interest for conflict of interest review.
If this activity meets the definition of an Outside Commitment, it should be disclosed for conflict of commitment review. The Outside Commitment Decision Tree on our Disclosure Requirements webpage may be of assistance in making this determination.
When submitting a proposal that may require additional space, renovations, or enhancements to utilities (like electrical, plumbing, or mechanical systems), it is crucial for investigators to reach out to Planning, Design & Construction (PD&C) and/or Facilities Management (FM) three months prior to the submission deadline. Such projects can involve significant costs and require additional lead time. By consulting the appropriate units well in advance, investigators can obtain cost estimates to include in their proposal budget whenever possible, and/or request and obtain commitments for alternate sources of funding prior to proposal submission. This ensures that the necessary funds and approvals are in place when awards are received so they can be accepted without delay.
Answer this question Yes if additional space, renovations, or changes in electrical, mechanical, or plumbing systems will be needed to conduct the project.
When answered Yes, a Questionnaire for "Space, Renovation" will be created requiring answers to additional questions:
What is the proposed location of additional space, renovations, or changes in electrical, mechanical, or plumbing systems? Be specific to avoid proposal delays (e.g. building name, room, total square feet, potentially impacted utilities systems, estimated cost, etc.).
In the text box, provide a brief summary of the anticipated needs. Be specific to avoid proposal delays and at a minimum provide the following: building name, street address, and room number if applicable; total square feet; potentially impacted utilities systems; cost estimate for changes.
Is the full cost of space changes or renovations included in the proposal budget?
Answer this question Yes if you have obtained a cost estimate and the full costs of the estimate are included in the proposal budget.
Answer this question No if you have not included the full costs of the cost estimate in the proposal budget or you have not obtained a cost estimate.
If not, What is the source of funds for space costs not included int he proposal?
If you answered No to the preceding question, in the text box enter specific amount(s), account number(s), and department(s)/unit(s). Documentation of alternate approved source(s) of funds should be attached in the Attachments section of the UAccess Research proposal.
Additional, more detailed guidance on estimating square footage, identifying which unit a space is assigned to, obtaining renovation estimates, submitting space change requests, and more can be found here.
Answer this question ‘Yes’ if this proposal involves companies or non-profits as partners if they are not already listed as a sponsor or prime sponsor. This would be the case for proposals where a company or nonprofit is a subrecipient, consultant, advisor, etc. If the answer is ‘Yes’ then it is required in the UAccess Research (UAR) proposal to list the full name(s) of any company or non-profit organization that will be involved as a partner, separated by commas.
Answer this question ‘No’ if the partner is already captured in the UAR proposal as the sponsor or prime sponsor.
Company and non-profit information helps RII to identify researchers, companies and non-profits interested in partnerships to strengthen responses to future sponsorship opportunities Contact Research Development Services (RDS) at ResDev@email.arizona.edu(link sends e-mail) for additional guidance about this question.
“Research Advancement Grants” describe the internal funding programs administered by RDI. Other departments or colleges on campus may also offer other internal funding programs. Frequently, the RDI Research Advancement Grants are discussed using the colloquial description of “Internal funding.”
Yes, a few are listed below.
- New Investigator Guide to NIH Funding, by the NIAID
- Early Stage and Early Established Investigator Policies, by the NIH
- Tips for New NIH Grant Applicants, by NIGMS
- Faculty Early Career Development Program (CAREER), by the NSFa
- NASA Science Directorate How-To Guide, by NASA
Answer this question ‘Yes' if the proposal involves handling any human derived products (tissue, saliva, organ, semen, vaginal secretions, feces, urine, blood, cells, etc.), or any other potential infectious materials (PIM) that could carry and transmit bloodborne pathogens. For assistance, contact Research Laboratory & Safety Services at (520) 626-6850 or rlss-bio-support@email.arizona.edu(link sends e-mail) or visit the or visit the Recombinant and Biohazardous Materials web page.
It depends on the country and the item. The U.S. government has export restrictions on certain items. Consult with Export Control to determine if your equipment, materials, data, or software is subject to these restrictions. Export Control will obtain licenses, exceptions or assist with other requirements to facilitate your travels, if required. Traveling with a “clean” laptop is recommended.
Yes. Each opportunity is reviewed independently.
Yes, unfunded research may be subject to export controls, particularly international collaborations.
You will be able to look up Negotations by department in the current version of UAccess Research (v. 5.2.1) until Thursday, January 20th before 5pm. After that, the old system will go dark. During system conversion, all negotiation records will be transferred over to the updated UAccess Research system. You will be able to look up Negotiations by department in the updated version of UAccess Research (Kuali SaaS) after go-live on Tuesday, January 25 after 8am.
To search for Negotiations by department in the updated version of UAccess Research, click on the Common Tasks button in the left-hand navigation panel. Find the Negotiation card and click Search Negotiation. Enter the Unit Number in the appropriate field and click search. You will only be able to open a Negotiation if you are provisioned to be able to do so for that unit.
As a National Cancer Institute Designated Comprehensive Cancer Center Facility, any and all use of the facilities of the Cancer Center must be reported to the National Institutes for Health (NIH) on an annual basis. This includes any use of Cancer Center Facilities, including use of the Common Equipment Rooms and/or the Shared Services of the Cancer Center. use of any lab, office, common equipment or shared services or any part of the Cancer Center Facility requires approval.
Answer this question ‘Yes’ for any proposal that involves research related to cancer. This information is compiled for the Arizona Cancer Center annual report.
This question was added to the UAccess Research (UAR) proposal in April 2020 to easily track and report on sponsored project proposals and awards related to COVID-19. Is the project scope of work related to COVID-19?
If you have any questions please contact sponsor@arizona.edu(link sends e-mail).
Generally, proposals to external sponsors require institutional review. If in doubt, contact Sponsored Projects and Contracting Services for clarification.
Yes. See the following link for detailed guidance on purchases and shipping. Depending on the item, export control laws and regulations may require security protocols (such as a TCP) to be in place before the item arrives on campus or is released for use. Items intended to be shipped outside the U.S. must be evaluated and coordinated by Export Control. If a license to export the item is required, Export Control will apply for such government authorizations. A customs broker may need to be involved in international shipments and purchases.
No, disclosures of "limited data sets" are not subject to the HIPAA accounting of disclosures requirements. The Department of Health and Human Services (DHHS) has taken the position that the privacy of individuals with respect to PHI disclosed in a "Limited Data Set" can be adequately protected through a single DUA.
No, a chemical spill kit may be shared between an Approval’s laboratories, as long as the laboratories are only separated by a single doorway (i.e. not a hallway). A reference guide for chemical spill kits is available on the RLSS website for more information.
In most cases, no budget is required for the pre-proposal. If a budget is required, a template will be provided as part of the pre-proposal application.
It depends, please visit the Types of Proposals webpage for further assistance.https://research.arizona.edu/research-administration/proposal-preparation/types-of-proposals#PreProposal
Full Committee or Expedite Research
The IRB must review and approve all changes to previously approved Full Committee or Expedite research prior to implementing the changes. For additional information, see our guidance on Modifying Approved Research.
More information on how to submit a Modification can be found on our eIRB Information webpage.
Exempt and Minimal Risk Research
Studies that are Exempt or Minimal Risk (MR) do not need to submit modifications to the IRB for review and approval unless the modifications change the nature of the project from being Exempt/MR. See the Exempt Minimal Risk Research guidance, which outlines when modifications are required.
If a modification is not required, the IRB recommends downloading the relevant approved documents within eIRB, making the necessary changes, and saving them separately in your research files. All modifications not reviewed by the IRB should be documented systematically to maintain an accurate record of all study activities to date.
Activities that meet the definition of Significant Financial Interest, Significant Personal Interest, Outside Commitment (including Foreign Components), Substantial Interest, Foreign Interest and Institutional Financial Interest need to be disclosed. Depending on the specific facts, that may include a foreign affiliation or presentation.
Please contact a member of OROI at coi@arizona.edu or 520-624-6406 to assistance.
No, you do not have to disclose your parent’s Outside Interests.
Federal regulations only require that you disclose you and your Family Member’s Outside Interests. Family Member means one's spouse, domestic partner and/or dependent child.
Yes, an IND application is a request to the FDA for authorization to administer an investigational drug (or biologic) to human or a marketed drug in a new indication and/or patient population. However, there are IND Exemptions. Please refer to the section on IND Exemptions for more information.
Key Persons should always have been added to the proposal and included in routing for review and approval of their role and commitment on the project. Unit Details had to be added manually and were not included or required for Key Persons previously and they were not included in Award Credit and F&A Revenue allocations except in special circumstances.
With this update, the Key Person's HR Home Unit will automatically be pulled in and they default to being included in Credit Allocations. You may simply enter 0% for Award Credit and F&A Revenue allocation for these individuals, or you may uncheck the box "Include in Credit Allocation" to remove them from allocations altogether.
Yes, all approvals in possession of radioactive material (including in storage and in waste) any time during a calendar month must perform a monthly survey unless the approval has been granted a specific approval condition exempting them from the requirement.
IRB review and approval is required for research projects involving human subjects.
Human subjects research is a systematic investigation (research question/hypothesis testing), designed to contribute to generalizable knowledge (add to the scientific knowledgebase in the field) done so while intervening or interacting with living individuals or their identifiable data. For additional information, please see our guidance on What is Human Research and watch our pre-recorded training video: Does Your Project Require IRB Review?
If you are still unsure whether or not your study is human subjects research, complete and submit the IRB Protocol for Determination of Human Research in eIRB.
No. As the administrative office that coordinates and manages the review of Limited Submissions, RDS does not assist in proposal preparation for pre-proposals. However, RDS does provide proposal support, when available, for all “tickets” awarded through the Limited Submission process.
Yes! The HSPP offers a variety of training opportunities including workshops and Office Hours. For training details, please visit the IRB Training Opportunities webpage. If you are interested in requesting training on a specific topic or having an HSPP member present at a class, lab, or faculty meeting, please contact the general inbox at VPR-IRB@arizona.edu.
Yes. The University of Arizona, as outlined in its Export Control Policy, is committed to complying with U.S. export controls laws and regulations that apply to its activities, including the International Traffic in Arms Regulations (ITAR), the Export Administration Regulations (EAR), and the Office of Foreign Assets Control (OFAC) regulations. All individuals affiliated with the University who work with, or have access to, export-controlled technical data, information, materials, and equipment are required to be familiar with and fulfill the requirements of the U.S. export controls laws and regulations by following applicable University policies and procedures
It depends on the equipment and its classification; contact export control for further review.
Occasionally some funding opportunities will be managed as Institutionally Coordinated Submissions and will appear on the Limited Submission table with that designation. Usually, these opportunities are for large and/or complex funding opportunities that require institutional support, core facilities, or leadership approval. To guarantee the submission of the strongest proposal(s) to the funder, RDS coordinates the institutional UA response.
Enter as many Facilities & Administrative rates as will be applicable to a proposal separated by a forward slash and without the "%" symbol. Example: 53/53.5
If you have any questions please contact sponsor@arizona.edu(link sends e-mail).
Select the appropriate rate category from the drop-down list in the UAccess Research (UAR) proposal. Visit the Facilities & Administrative (F&A) Rates page of the Research Support website and view the F&A rate table for additional guidance.
For further questions, please contact sponsor@arizona.edu(link sends e-mail).
Rate Categories:
- Federal negotiated rate: It is the University of Arizona’s policy to request the appropriate federally negotiated F&A rate for all sponsored activity regardless of funding source, the federally negotiated F&A rate will be the basis for budget purposes on all sponsored activity. When funding flows from a federal prime sponsor to the University of Arizona, through a pass-through entity, as in the case of federal flow-through awards, the University will accept stipulations that meet the requirements of Uniform Guidance 2 CFR 200.414
- Sponsor F&A rate stipulation: If a sponsoring agency limits or forbids the reimbursement of F&A based on federal/state/local law, administrative regulation, or published sponsor policy, exceptions for Federal Flow through or Stipulated F&A Rates apply. Documentation for a stipulated rate must be included with the proposal. To document a stipulated rate, attach a copy of the law, regulation or published policy stating the limitation on F&A reimbursement under the Attachments section of the UAccess Resarch proposal document as attachment type "F&A Stipulation".
- Other standard UA F&A rate: Other standard UA F&A rates are described in the standard F&A rate table on the Facilities & Administrative (F&A) Rates page.
- F&A waiver (rarely approved, attach waiver request template): The decision to grant or deny a waiver request is at the sole discretion of UArizona Research Innovation, & Impact (RII) but is in concurrence with the approval from the representative Department Head, and Dean or Associate Dean for Research. These offices will work with RII to review and obtain the approval in advance of a UAR application or contract. All requests must use the F&A Waiver Request Template with the Department Head and Dean or Associate Dean for Research approval and must be submitted with a UAR proposal no less than five working days in advance of the deadline. Attach the Template in the UAR proposal Attachments section as attachment type "F&A Waiver Request Template."
As of July 2024 this UAccess Research (UAR) proposal option is no longer in use.
If you have any questions please contact sponsor@arizona.edu(link sends e-mail).
FDA/EPA Quality Assurance (GLP/cGMP/QA)?
Answer this question ‘Yes’ if the proposal involves any of the terms below or requires adherence to any FDA/EPA quality assurance program. If the project you are proposing involves the manufacture of a medical/therapeutic product, the evaluation of an FDA grant “Test or Control Article”, or collaboration with another researcher/institution upon research requiring FDA/EPA quality assurance program enrollment please contact Research Laboratory & Safety Services at rlss-help@arizona.edu. Visit the Good Laboratory Practices web pages for additional guidance.
At this time, the RLSS maintains a voluntary GLP preparation program (training & inspections), the UArizona has not constituted an institutional Quality Assurance Unit and would need reasonable time to do so to accommodate anyone with an FDA/EPA quality assurance program requirement.
- Good Laboratory Practices
- Good Manufacturing Practices
- Quality Assurance
- 21CFR
- Title 21
- EPA Quality Program
- GLP
- GMP
- cGMP
- Test Article
- Control Article
Enter the account number of a past sponsored project if the current proposal may be funded as a new and separate award but is the next phase or a continuation of the past project.
If you have any questions please contact sponsor@arizona.edu(link sends e-mail).
Answer this question ‘Yes’ if any portion of the project is to be completed with the assistance of collaborators in a foreign nation and use the lookup feature in the UAccess Research (UAR) proposal to select the country of each foreign collaborator.
Answer this question ‘Yes’ if the proposal involves the use and storage (including cleaning and sterilizing of equipment) of any quantity of any solvents, oxidizers, corrosives, compressed gases, cryogenics, heavy metals, dust-generating compounds, ATF regulated materials, DEA Controlled Substances, pesticides, fertilizers, and/or hormones/steroids. Visit the Chemical Safety Program web page for additional guidance or contact Research Laboratory & Safety Services at rlss-help@arizona.edu(link sends e-mail) with questions.
Examples include but are not limited to:
- Ethanol
- Methanol
- Tetrahydrofuran
- Acetone
- Potassium permanganate
- Hydrogen peroxide
- Sodium/Calcium hydroxide
- Sulfuric/Nitric/Acetic acid
- Carbon dioxide
- Argon
- Liquid nitrogen
- Arsenic
- Mercury
- Mine tailings
- Silica powder
- Praxair FE-271 (or other brand additive manufacturing metal powder product)
- Nitrotriazolone (NTO)
- Nitrocellulose
- Ketamine
- Testosterone
- Cannabidiol (CBD)
- Round-Up (or other brand glyphosate product)
- Microthiol (or other brand sulfur fungicide product)
- Pyrethroids
- Novel mode-of-action insecticide
- Urea
- Mono-ammonium phosphate (11-52-0)
- Iron/Zinc/Molybdenum chelates
- Zoetis (or other brand estradiol product)
- Corticosteroids
Conflict of interest (COI) and conflict of commitment (COC) reviews are separate because COI relates to bias in decisions and COC relates to a University employee's time & effort for UArizona, UArizona resources and UArizona Assets.
Sponsors & funders have identified the following concerns:
1. conflicts of interest
2. shadow labs
3. loss of Intellectual Property
4. conflicts of commitment, including theft of time, resources and assets
A HIPAA Covered Entity, or a Hybrid Covered Entity like UA, may use a member of its own workforce to create the "Limited Data Set." Alternatively, the recipient may create the "Limited Data Set," so long as the recipient is acting as a Business Associate or Subcontractor (pursuant to a Business Associate Agreement) of the Covered Entity or Hybrid Covered Entity.
There are several portals and databases that can be used to search for potential collaborators. For more information on these as well as collaboration spaces, centers and institutes, core facilities, and other tools and resources, visit the Collaboration section of the Research Gateway.
If you work with an investigator who has a preferred name in addition to their primary legal name, and who may have had proposals, awards, negotiations or subawards under both names, use the vertical line ‘or’ operator, “|”, in lookup screens accessed via Common Tasks in the UAR left hand navigation pane. This will allow a search for all names the person might have used in past records.
In the past, UAR only populated a person’s legal/primary name as it was entered into the UAccess Employee system. As of the January 25, 2022 UAccess Research (UAR) system update, both primary legal name and preferred name are brought into UAR, with the default being preferred name. As a result of added flexibility, people who have a preferred name or who have changed their name over time may have older records under a different name.
Example: john den|ja*den finds John R Den, Johnna Alden, OR Jack Den, Jason Hayden.
Lookup Wildcards
|
Operator |
Name |
Compatible Data Types |
Precedence |
Notes |
|
| |
Or |
All |
Always |
|
|
&& |
And |
All |
Always |
|
|
! |
Not equal to |
String |
1 |
If used repeatedly, an && is assumed. Ex: !1490!1491 is like !1490&&!1491 |
|
?, * |
Like |
String |
7 |
? matches one character. |
|
.. |
Greater than or Equal to and Less than |
String, Number, Date |
2 |
|
|
> |
Greater than |
String, Number, Date |
3 |
|
|
< |
Less than |
String, Number, Date |
4 |
|
|
>= |
Greater than or equal to |
String, Number, Date |
5 |
|
|
<= |
Less than or equal to |
String, Number, Date |
6 |
|
-
limiting what you take abroad;
-
keeping information in your possession or locked in a secure location;
-
using a “clean” laptop – with minimal information;
-
using the university VPN if you need to access data;
-
encrypting your device; and
-
screening collaborators in advance.
Non-U of A Investigators should follow the steps on this webpage: Information for Non-UA Subcontractors, Consultants, and Collaborators.
Visit the Build a Budget section of the Research Gateway to get started, and contact your departmental/unit business office for assistance.
Click on Common Tasks in the left-hand navigation panel. Find the Proposal Development card and select Create Proposal.
See the Create and Save a New Proposal video for step-by-step instructions. Be aware that there are additional videos for completing each section of the Proposal Development document and submitting the proposal to routing for review, approval, and submission.
Stock and equity only need to be disclosed if they are an Investigator's Significant Financial Interest or a University Employees Substantial Interest.
A Significant Financial Interest includes:
-
Any equity in a private company, regardless of value
-
Equity valued at $5,000 or more in a public company
However, income from investment vehicles such as mutual funds and retirement accounts, as long as the Investigator does not directly control the investment decisions made by the investment managers within these funds or accounts, do not require disclosure.
A Substantial Interest includes: The ownership of three percent or more of the shares of a corporation for profit, where the total annual income from dividends, including the value of stock dividends, from the corporation exceeds five percent of the total annual income of the employee.
Completing the Stock in External Entity Page in eDisclosure
On the Stock or Equity page in eDisclosure, there are two options for disclosing stock and equity:
- "Do you own stock / partnership shares in this organization?" should be used to disclose (1) stocks and shares that you own in a public entity and (2) partnership agreements/shares.
- "Do you own stock options or any other form of equity in this organization?" should be used to disclose (1) other ownership interests (e.g., equity in a private entity), (2) stock options, including Employee Stock Options ("ESOs"), and stock warrants.
Stock & Equity Definitions
- Equity is any stock or other ownership interest, or an entitlement to obtain any stock or ownership interests (e.g., stock options and warrants). The value of Equity is determined through reference to public prices or other reasonable measures of fair market value (e.g., assets - liabilities = value).
- “Exchange-traded funds (ETFs) are SEC-registered investment companies that offer investors a way to pool their money in a fund that invests in stocks, bonds, or other assets. In return, investors receive an interest in the fund. Most ETFs are professionally managed by SEC-registered investment advisers. Some ETFs are passively-managed funds that seek to achieve the same return as a particular market index (often called index funds), while others are actively managed funds that buy or sell investments consistent with a stated investment objective. ETFs are not mutual funds.” (https://www.investor.gov/introduction-investing/investing-basics/glossary/exchange-traded-fund-etf, last visited Oct. 27, 2022)
- “Options are contracts giving the purchaser the right – but not the obligation -- to buy or sell an underlying asset at a fixed price within a specific period of time. Stock options are traded on a number of exchanges.” (https://www.investor.gov/introduction-investing/investing-basics/glossary/options, last visited Oct. 27, 2022)
- Partnership Shares is a partnership arrangement between two or more people to oversee business operations and share its profits and liabilities. (See generally https://www.irs.gov/businesses/partnerships, last visited Oct. 27, 2022)
- “Stocks are a type of security that gives stockholders a share of ownership in a company. Stocks also are called ‘equities.’” (https://www.investor.gov/introduction-investing/investing-basics/investment-products/stocks, last visited Oct. 27, 2022)
- A Stock Warrant “is a contract that gives the holder the right to purchase from the issuer a certain number of additional shares of common stock in the future at a certain price, often a premium to the stock price at the time the warrant is issued.” (https://www.finra.org/investors/insights/spac-warrants-5-tips, last visited Oct. 27, 2022)
See Also
We recommend that you take a proactive role in identifying relevant funding opportunities. To assist you, we have provided helpful resources including a list of hand-curated funding opportunities for Early Investigators, and a compilation of search databases, funding sources, and distribution lists.
The action level for contamination is 50 counts per minute (cpm) above the background count rate.
Prospective Approval Holders register their laboratory or set of laboratories in the Laboratory Chemical Safety Program by completing the New Lab Registration and Assessment. You will be contacted by the RLSS within 3 business days after registration submission to schedule an initial visit.
Investigators are required to report local problems, concerns, serious risks, and failure to follow the protocol to the IRB for all human subjects research. These reports must be submitted to the IRB within ten (10) business days of discovery. Changes made to eliminate risk to subjects must be reported to the IRB within five (5) business days of discovery. Reportable items must be submitted in eIRB as a Reportable New Information (RNI). If the University of Arizona IRB is the IRB of Record for another site, the site must follow the same requirements. For additional information, please see our guidance on Reporting New Information.
Subscribe to the Limited Submissions Newsletter, the RDS weekly digest of funding opportunities and news which lists upcoming and open funding opportunities.
All Limited Submission opportunities currently in a competition will be listed in Arizona Cultivate. You can log in and apply using your NetID. Most applications require both textbox entry of application information such as a summary, a significance statement, approach, and expected outcomes, as well as uploads in PDF format. RDS recommends logging in to the system to see what is required well in advance of the internal submission deadline.
The value of Equity is determined through reference to public prices or other reasonable measures of fair market value (e.g., assets - liabilities = value).
Stock in a public entity is valued based on Annual Reports and other public valuations.
Valuing stock in a private entity can be done various ways using methods such as valuation ratios, internal rates of return, comparative analysis and discounted cash flow analysis.
See Also
The purpose of the OFAC regulations is to enforce embargoes and economic sanctions. In general, the OFAC regulations prohibit exports to certain sanctioned/embargoed countries such as Iran, Cuba, Sudan, North Korea, and Syria.
OFAC considers providing anything of value or a service to Iran or the government of Iran would require prior government approval. For example, giving a professional presentation, even if it does not contain materials controlled under ITAR or EAR, is deemed under OFAC to be a “service” and “something of value” provided to the recipient audience.
In addition to the points listed above there are other considerations which vary by country:
- Attending a conference in Iran (OFAC considers this to be an “import”) or speaking at a conference in Iran (providing a service or something of value) requires a license. An OFAC license for Iran generally takes six to nine months (or longer) to process once submitted.
- Participating in certain online courses abroad requires an OFAC license, if the student is ordinarily a resident of sanctioned country (Cuba, Iran, Syria, or the Crimea Region of the Ukraine).
- Any technical discussions, formal or informal, could require a license and would be prohibited prior to the receipt of the necessary license(s).
- Travel to Cuba has special considerations. For information on Cuba travel, see http://www.treasury.gov/resource-center/sanctions/Programs/Pages/cuba.aspx.
- The University of Arizona will NOT apply for an OFAC License for activities in or with Syria - no University travel to Syria will be approved. Travel to Iran will be approved on a case-by-case basis and only upon receipt of any required OFAC or other government licenses.
If an employee travels to any sanctioned country on their own time, the individual may not take or send anything university-owned such as equipment, software, technology, or data, or represent the university in any capacity.
The University of Arizona’s Export Control team works closely with the Contracting Office to identify contracts with NIST requirements or clauses with publication restrictions (e.g., DFARS 252.204-7012 and 252.204-7000). Export Control is also alerted when there are similar safeguards/restriction clauses in contracts that are not sponsored by Department of Defense (NASA contracts often have similar clauses).
An export control checklist is used in the evaluation process. The three-part checklist must be completed by the PI, Contracting Office, and Export Control. The checklist highlights DFARs clauses in addition to potential export control red flags.
UA faculty, staff, and students traveling internationally on behalf of UA for business, research, or other purposes are required to register well in advance of their departure (travel.arizona.edu). In addition to obtaining UA approval, the traveler may require a license, license exception/exemption, or other guidance to hand-carry items abroad, access data, interact with certain persons, speak at a conference, conduct research, provide training or other services, or engage in other UA related activities.
See the Export Control resource on international activities for additional guidance.
Export Control applies for all export control licenses on behalf of the University. NOTE: Obtaining an export license may take several months and there is no guarantee that the U.S. government will approve a license request.
Prior to travel, to avoid collaborating with a prohibited party, foreign parties should be screened using the Restricted Party Screening tool. Export Control or your Department Administrator can assist with conducting screenings.
What is Arizona’s COI Law?
In addition to federal conflict regulations, the University of Arizona must also comply with Arizona’s conflict of interest (COI) law.
A Substantial Interest is any nonspeculative pecuniary or proprietary interest, either direct or indirect, other than a remote interest. Remote interest is defined in A.R.S. § 38-502(10).
To mitigate the possibility that a personal influence might bear upon a University employee’s decision in his or her capacity as a public employee, a University employee who has, or whose Relative has, a "Substantial Interest" in
- any contract, sale, purchase, or service by or to the Arizona Board of Regents (“ABOR”) or UArizona, or
- any decision of ABOR or UArizona,
the University employee shall refrain from voting upon or otherwise participating in any manner as a University employee regarding such contract, sale, purchase, service or decision.
What do I need to do?
University employees must disclose all substantial interest in the official records of ABOR. UArizona’s Conflicts of Interest & Commitment Policy complies with this law by requiring disclosure in eDisclosure.
What is considered when making Substantial Interest determinations?
- Will the contract, sale, purchase, service, or decision have an impact, either positive or negative, on an interest of a University employee or their Relative?
- Is the interest pecuniary (involves money) or proprietary (involves ownership)?
- Is the interest a remote interest?
How is this state law applicable to Research and Startup Companies?
A University employee who has, or whose Relative has, a Substantial Interest in an entity cannot (1) participate as a University employee in contracting and purchasing decisions related to the entity or (2) subaward research to the entity. This includes the process leading up to the decision (e.g., making recommendations, giving advice, communicating with anyone involved in the purchasing process).
Who is a Relative?
Like we do with federal conflict regulations, the University relies on the state law to define Relative. Thus, Relative has the meaning set forth in A.R.S. 38-503 (i.e., one's spouse or domestic partner, child grandchild, grandparent, sibling and their spouse or domestic partner, half-sibling and their spouse or domestic partner, and the parent, sibling or child of a spouse or domestic partner).
Even if the University employee does not have a substantial interest in a decision in which they are about to participate, if one of their Relatives has a substantial interest in the decision, they must disclose the interest and refrain from participating in the decision.
Noncompliance with this law cannot be justified by stating you are not unaware of your Relative’s interest. Public officers and employees have an affirmative obligation to become aware of any interests their relatives may have that may create a Substantial Interest.
Who is the Conflict Official for Arizona’s COI law?
While Arizona’s COI law is codified in the Conflicts of Interest & Commitment Policy and Substantial Interest disclosures are made through eDisclosure, Ted Nasser, Chief Procurement Officer, is the Conflict Official for Substantial Interests.
What if I have a question about Substantial Interests?
Please contact the Office for Responsible Outside Interests at coi@arizona.edu for questions related to Substantial Interests. This law is broadly construed in favor of the public and substantial civil and criminal penalties are provided for failure to comply with the statutory requirements. It is imperative that your questions are answered.
“Remote interest” means:
- That of a nonsalaried officer of a nonprofit corporation.
- That of a landlord or tenant of the contracting party.
- That of an attorney of a contracting party.
- That of a member of a nonprofit cooperative marketing association.
- The ownership of less than three percent of the shares of a corporation for profit, provided the total annual income from dividends, including the value of stock dividends, from the corporation does not exceed five percent of the total annual income of such officer or employee and any other payments made to him by the corporation do not exceed five percent of his total annual income.
- That of a public officer or employee in being reimbursed for his actual and necessary expenses incurred in the performance of official duty.
- That of a recipient of public services generally provided by the incorporated city or town, political subdivision or state department, commission, agency, body or board of which he is a public officer or employee, on the same terms and conditions as if he were not an officer or employee.
- That of a public school board member when the relative involved is not a dependent, as defined in section 43-1001, or a spouse.
- That of a public officer or employee, or that of a relative of a public officer or employee, unless the contract or decision involved would confer a direct economic benefit or detriment on the officer, the employee or his relative, of any of the following:
- Another political subdivision.
- A public agency of another political subdivision.
- A public agency except if it is the same governmental entity.
- That of a member of a trade, business, occupation, profession or class of persons consisting of at least ten members which is no greater than the interest of the other members of that trade, business, occupation, profession or class of persons.
- That of a relative who is an employee of any business entity or governmental entity that employs at least twenty-five employees within this state and who, in the capacity as an employee, does not assert control or decision-making authority over the entity's management or budget decisions.
The ownership of any publicly traded investments that are held in an account or fund, including a mutual fund, that is managed by one or more qualified investment professionals who are not employed or controlled by the officer or employee and that the officer or employee owns shares or interest together with other investors.
Approval timelines are contingent upon the complexity of a study, the investigator's responsiveness, and whether applicable approvals have been obtained. Average approval timelines are broken down by level of review:
- Non-Committee Review (Minimal Risk/Exempt/Expedite): 14 business days
- Committee Review: 30 business days
Survey records must be maintained for three years after the date of the survey.
There is no set number of areas required to be surveyed, but you must ensure that all radioactive material use areas within the lab are covered.
The information contained in a limited submission pre-proposal and the associated supplemental documents is considered highly confidential and all efforts will be made to ensure the fair, objective, and confidential review of each pre-proposal. Review panel members are required to sign a conflict of interest statement prior to pre-proposal review and to adhere to strict guidelines to ensure confidentiality of the content of all limited submissions applications.
Answer this Hispanic Serving Institution (HSI) question 'Yes' if the proposal falls into one of the three categories described below.
HSI Grant requests for proposal (RFPs) generally fall into three categories:
HSI Required: These funding opportunities are exclusive to HSIs and require proof that the university is an HSI. A digital copy of proof from the U.S. Department of Education can be provided upon request. Direct inquiries to Riley McIsaac, rmcisaac@arizona.edu.
Minority Serving Institution (MSI) Required: These funding opportunities are not exclusive to HSIs, but instead require that a submitting institution have MSI status (HSIs are included under the umbrella of MSIs). Proof of HSI status may or may not be required. Minority or underrepresented students (e.g., first generation, Pell grant recipient/low income) and/or communities/populations are often expressed as the targeted populations of interest.
Intentionally Involves Minority Students and/or Communities/Populations: These funding opportunities are not exclusive to HSIs or MSIs, but they explicitly call for or encourage the engagement of minority or underrepresented students, and/or communities/populations. Engagement of these students/communities/populations should be coupled with asset-based recruitment strategies, culturally relevant learning experiences, inclusive mentoring practices, and much more.
For questions or to learn more, please contact Riley McIsaac, Associate Director of Grants Development in the Office of Hispanic Serving Institutions (HSI) Initiatives (rmcisaac@arizona.edu).
Visit HSI Initiatives at: https://hsi.arizona.edu/.
The University of Arizona was federally designated as a Hispanic Serving Institution in Spring 2018, having reached the 25% undergraduate Hispanic enrollment requirement. UArizona was the first four-year public university in the state of Arizona to become an HSI and one of 16 R1 HSIs across the nation.
Answer this question ‘Yes’ if human subjects are involved in the proposal. The University is required to safeguard human participants that are involved in research projects. For any project involving the use of human participants, a protocol must be submitted to the University’s Human Subject Protection Program (HSPP) and the Institutional Review Board (IRB) for review and approval.
The HSPP and IRB ensure that Human Subjects rights and welfare are protected, the risk and potential benefits are weighed accurately, subject selection is fair and that the participants have an informed consent. Approval is required before any work with Human Subjects is initiated.
Visit the Human Subject Protection Program section of the Research Support website for additional guidance.
Below, please find a list of the potential points of disclosure.
COC Form – Outside Activity
If your seminars meet the definition of Outside Commitment, you should submit a COC form in eDisclosure to get prior approval to engage in the Outside Activity.
Outside Commitments: (1) are professional and other activities that are related to a University Employee’s professional expertise, outside of their University duties and responsibilities; (2) are for the benefit of an external entity or individual and are not covered by a fully executed written agreement between the University and the external entity; and (3) require a time commitment. Outside Commitments include Outside Employment, independent contracts for consulting services, private consulting groups comprised of University Employees, volunteer/pro bono work, appointments at postsecondary educational institutions, and foreign components, as that term may be updated by the University’s Office for Responsible Outside Interests.
You can also use this decision tree to determine if the activity is an Outside Commitment: Outside Commitment Decision Tree.
COI Disclosure – Foreign Interests
If you do end up receiving a payment of any amount, an in-kind contribution or Other Support, it must be disclosed as a Foreign Interest.
COI Disclosure – Foreign Travel
PHS and Dept of Energy Investigators only: Must disclose any reimbursed or sponsored travel related to your institutional responsibilities, regardless of the amount, unless received from a Federal, state, or local government agency of the United States; a domestic Institution of Higher Education; or a domestic research institute that is affiliated with a domestic Institution of Higher Education.
COI Disclosure – Foreign Affiliations
As part of your eDisclosure submissions, you must disclose whether you have a foreign affiliation.
Below, please find a list of the potential points of disclosure.
COC Form – Outside Activity
If any of your activities during your travel meet the definition of Outside Commitment, you should submit a COC form in eDisclosure to get prior approval to engage in the Outside Activity.
Outside Commitments: (1) are professional and other activities that are related to a University Employee’s professional expertise, outside of their University duties and responsibilities; (2) are for the benefit of an external entity or individual and are not covered by a fully executed written agreement between the University and the external entity; and (3) require a time commitment. Outside Commitments include Outside Employment, independent contracts for consulting services, private consulting groups comprised of University Employees, volunteer/pro bono work, appointments at postsecondary educational institutions, and foreign components, as that term may be updated by the University’s Office for Responsible Outside Interests.
You can also use this decision tree to determine if the activity is an Outside Commitment: Outside Commitment Decision Tree.
COI Disclosure – Foreign Interests
If you do end up receiving a payment of any amount, an in-kind contribution or Other Support, it must be disclosed as a Foreign Interest.
COI Disclosure – Foreign Travel
PHS and Dept of Energy Investigators only: Must disclose any reimbursed or sponsored travel related to your institutional responsibilities, regardless of the amount, unless received from a Federal, state, or local government agency of the United States; a domestic Institution of Higher Education; or a domestic research institute that is affiliated with a domestic Institution of Higher Education.
COI Disclosure – Foreign Affiliations
As part of your eDisclosure submissions, you must disclose whether you have a foreign affiliation.
Below, please find a list of the potential points of disclosure.
COC Form – Outside Activity
If your visits or seminars to Japanese universities meet the definition of Outside Commitment, you should submit a COC form in eDisclosure to get prior approval to engage in the Outside Activity.
Outside Commitments: (1) are professional and other activities that are related to a University Employee’s professional expertise, outside of their University duties and responsibilities; (2) are for the benefit of an external entity or individual and are not covered by a fully executed written agreement between the University and the external entity; and (3) require a time commitment. Outside Commitments include Outside Employment, independent contracts for consulting services, private consulting groups comprised of University Employees, volunteer/pro bono work, appointments at postsecondary educational institutions, and foreign components, as that term may be updated by the University’s Office for Responsible Outside Interests.
You can also use this decision tree to determine if the activity is an Outside Commitment: Outside Commitment Decision Tree.
COI Disclosure – Foreign Interests
If you do end up receiving a payment of any amount, an in-kind contribution or Other Support, it must be disclosed as a Foreign Interest.
COI Disclosure – Foreign Travel
PHS and Dept of Energy Investigators only: Must disclose any reimbursed or sponsored travel related to your institutional responsibilities, regardless of the amount, unless received from a Federal, state, or local government agency of the United States; a domestic Institution of Higher Education; or a domestic research institute that is affiliated with a domestic Institution of Higher Education.
COI Disclosure – Foreign Affiliations
As part of your eDisclosure submissions, you must disclose whether you have a foreign affiliation.
Existing programs or centers with no break in funding (i.e. successful prior renewals or first competitive renewal) will have the opportunity to recompete to the sponsor without internal competition for two submission cycles. If, however, the existing program or center is unsuccessful in obtaining its renewal after two consecutive submissions, an internal limited submission competition will occur. If other pre-proposals are received for the limited submission competition, it is not guaranteed that the PI of the existing programs or centers will be granted the ticket.
It depends. First, check the RDS Limited Submissions Table to see if the funding opportunity is listed and currently accepting internal pre-proposals. If an internal competition is underway, review the pre-proposal requirements and submit your pre-proposal. If the funding opportunity is not listed, email LimitedSubmissions@email.arizona.edu to express your interest in applying. RDS will establish eligibility to apply based on the Preferred Timeline for Limited Submissions.
If you are an Investigator, you must disclose, via eDisclosure, affiliations with foreign governments, foreign institutions of higher education, any other foreign entity and foreign nationals when conducting activities such as consulting, laboratories or teaching (whether paid or unpaid, or voluntary). Gifts and/or any travel support of any amount you receive must also be disclosed.
Additionally, all University Employees whose FTE is 0.50 or greater, are required to submit a disclosure of Outside Commitments and/or Outside Employment (COC form) via eDisclosure for any activity that meets the definition of an Outside Commitment or Outside Employment. The Outside Commitment Decision Tree may be of assistance to you in making the determination as to whether this activity meets the definition of an Outside Commitment.
See Also
Contact RDS during normal business hours (M-F, 9a-5p) at 621-8585 or email ResDev@email.arizona.edu for assistance using Arizona Cultivate or for questions regarding any Limited Submission internal competition.
Please visit our Getting Started webpage.
In some proposals on the Dashboard you will see a small red Compliance pill: . There is no action required on these proposals. It's simply a visual indicator that the project involves Human Subjects or live Vertebrate Animal Subjects. If you click on the Compliance pill you will see what Type (Animal Subjects or Human Subjects) and Status (Exempt, Not Yet Applied, Pending) were included in the proposal.
The HSPP does not accept modifications to determinations of human research as these projects are not overseen by an IRB. Investigators may proceed as long as the intent behind the project remains unchanged. If changes are made affecting whether the activities are human research, a new determination request must be submitted for review in eIRB.
Contact RDS as soon as possible so other potential applicants can move forward with a submission to the funder. Certain limited submissions are very competitive at UA, e.g. the National Science Foundation (NSF) Major Research Instrumentation (MRI) program. If you receive the ticket to be the institutional submission for a competitive limited submission opportunity (i.e. an opportunity where there was competition for the permission) and you choose not to submit and you do not notify us within four weeks of the sponsor deadline of your decision, you will be ineligible to reapply in the following cycle.
RDS makes every effort to have pre-proposals reviewed in a timely manner, usually within two weeks of the internal deadline. Our goal is to maximize the external proposal preparation time for selected researchers to submit to the sponsor for funding.
University Employees whose FTE is 0.50 or greater, are required to disclose any activity that meets the definition of an Outside Commitment or Outside Employment by submitting a disclosure of Outside Commitment or Employment (COC form) via eDisclosure. University approval is required prior to full-time University Employees (0.50 FTE or greater) entering into an Outside Commitment or Outside Employment (regardless of whether or not compensated for the activity).
- The Outside Commitment Decision Tree on our Disclosure Requirements webpage link may be of assistance to you in determining whether the activity meets the definition of an Outside Commitment. More information on Outside Activity can also be found on our website.
Additionally, as an Investigator, one of the requirements is disclosure of Outside Interests (Significant Financial, Significant Personal and Foreign Interests). You can find an overview of the Disclosure Requirements on our website including Disclosure Table resources which outlines the disclosure guidelines.
Consulting Agreements
Our website has information related to Consulting Agreements including a Consulting Agreement Addendum that the University developed as a resource that University employees can use as an addendum to any agreement that you sign or can be used as guidance on common issues that may arise in a consulting arrangement that may relate to your university employment.
Preproposals are archived within Arizona Cultivate and are not distributed or made publicly available.
Once you have received the “ticket” you should work with your department administrator and, if available, the RDS Associate designated to support the proposal development. All limited submissions submitted to an external funding agency or sponsor are handled through Sponsored Projects and Contracting Services.
Typically, U of A researchers must receive U of A IRB approval to conduct research with human subjects, regardless of where the research takes place. When collaborative projects are expected to involve researchers from multiple institutions, contact the HSPP to determine next steps. While in some cases each investigator should work with their own institution’s IRB, in other cases, it might be advisable (or required by a funder) to arrange an IRB reliance/institutional agreement (IAA) to designate one IRB to review and approve the research as a whole. Visit the Single IRB Research webpage for details.
This should be disclosed as a Foreign Interest by selecting “Remuneration or Reimbursement (excluding sponsored travel)” and “Sponsored or reimbursed travel.” For Foreign Interests, there is no dollar threshold.
Foreign Interests are:
- Participation in a foreign talent or similar-type program
- All resources and other support, both domestic and foreign, for ongoing research projects, including those conducted at a different institution
- In-kind contributions from domestic and foreign institutions or governments that support your research activities
- Any payment, reimbursement, travel support or other compensation, of any amount, that you personally receive, or will personally receive, from a foreign entity
The HSPP considers Children, Cognitively Impaired Individuals, Pregnant Women and Neonates, Prisoners, and Native American or International Indigenous Populations to be vulnerable populations that require additional protections and safeguards. When your targeted study population includes one or more of these vulnerable populations, the appropriate Appendix for Vulnerable Populations is required with your eIRB submission. Please see our guidance on conducting research with these populations.
For specific questions regarding research involving Native American or International Indigenous Populations, contact Claudia Nelson at cen@arizona.edu.
If you are traveling outside of the United States to conduct human subjects research, you will need to register your travel with the UA Travel Registry. For more information on conducting international research, see our guidance on International Research.
Prior to IRB submission, all projects that will utilize Banner Health resources (i.e., facilities, patients, employees) must be entered into the University of Arizona Health Sciences (UAHS) Research Administration Portal (RAP). Upon submission to the IRB, please upload RAP approval to eIRB. In addition, the IRB also requires that the UA Medical Consent Form be used when Banner Health resources will be utilized.
Yes, you will need both a Data Use Agreement (DUA) and Business Associate Agreement (BAA) because the Covered Entity or Hybrid Covered Entity (UA) is providing the recipient with PHI that includes direct identifiers. For that reason, a BAA would be required to disclose the direct identifiers to the recipient. Once the Limited Data Set is created under the BAA, all of the PHI, other than the PHI qualifying as the limited data set under the DUA, must be returned to UA.
As part of the system conversion, all current access and roles with the exception of Lead-Unit pre-approval will be migrated over to the updated system environment. There is no action required on your part.
The JoinCostShare node will always display in the Current Route Node(s) regardless of whether a budget is included with cost share units as it allows the cost share workflow functionality.
Yes. As long as an original document was uploaded as an attachment prior to routing, the proposal initiator can replace the attachment with an updated/revised version by navigating to the Attachments section and clicking the Details button on the right side of the page next to the desired attachment. The initiator will then choose the new file to upload and click Save.
Depending on your destination(s), authorization from the U.S. Treasury’s Office of Foreign Assets Control (OFAC) may be required. Travel to an embargoed/sanctioned country (e.g., Cuba, Iran) may require prior authorization in the form of a license. If travel to an embargoed country is for personal reasons, no University equipment may be taken, and no University business should be conducted without prior authorization. Most activities involving Iran (even remotely) will require a license.
Yes. The content included/shown on the Medusa page is the same in the updated UAccess Research System. It just has a different look and feel.
You will still have the options of displaying Proposal > Award where the Proposal is the top item and all awards, negotiations, and subawards associated with the proposal fall under it, or Award > Proposal where the Award is the top and all proposals, negotiations, and subawards associated with the award fall under it. You can expand each item by clicking on it, and have the option to open each item directly from the Medusa page (as long as you are provisioned to view that item).
RDS publishes the name of the PI selected for each Limited Submission opportunity on the Limited Submission Table on the Research Gateway. RDS seeks to coordinate Limited Submissions for the university to facilitate the best proposal submission from an institutional perspective and encourages communication between all interested applicants to a limited submission opportunity. That said, the choice to collaborate is at the discretion of the PI/Team that receives the ticket to proceed.
No. Deadlines for Limited Submission internal competitions are treated the same as funding deadlines for Federal agency sponsors, Foundations or other external funding sources. These opportunities are advertised widely and well in advance of the application deadlines. To maintain fairness for all, the deadline is firm and consistent for all applicants without exception.
Answer this question ‘Yes’ if live vertebrate animals are involved in the proposal. A protocol must be submitted for approval to the Institutional Animal Care and Use Committee (IACUC) before an award is made, but not at the time of proposal.
IACUC oversees the university’s animal care and use programs. This unit ensures the humane and ethical treatment of the animals used in research, testing and education. IACUC reviews all requests to use vertebrate animals to ensure compliance with federal regulations.
Principal Investigators who plan to use animal subjects as a part of their research should contact a member of the IACUC early in the project design stage to determine appropriate species as models for research and appropriate procedures to be used in the course of research (from UHAP). Visit the Institutional Animal Care and Use Committee (IACUC) program pages of the Research Support website for additional guidance.
There are specific requirements for NSF proposals that may impact the resources or interests of a federally recognized Tribal Nation. Detailed guidance and FAQs for the University of Arizona process for NSF proposals can be found here.
Answer the Native or Indigenous research and/or engagement question ‘Yes’ if this proposal falls into one of the categories described below:
- the research or institutional engagement intentionally involves participation by members of a sovereign tribe or indigenous community and may foreseeably result in research results with implications specific to a tribe, indigenous community, or to individuals as members of the tribe or community. Note: Such engagement may occur with native or indigenous peoples outside the U.S.
- the research or institutional engagement takes place in Indian Country, or Alaska Native homelands, and/or on land under the control or jurisdiction of a sovereign tribe or indigenous community. Note: Such engagement may occur with native or indigenous peoples outside the U.S.
- human research is conducted in Indian Health Service (IHS) facilities or involving IHS staff or resources. Note: Additional engagement with the IHS, tribal Institutional Review Boards (IRBs), or other entities may be required.
- the research involves human subjects, including genetic testing or testing of blood, tissue, or other biological materials if the individual's membership in or affiliation with a tribe or indigenous community is identified, and that is intended to or may foreseeably result in conclusions or generalizations about a tribe, indigenous community, or individuals as members of the tribe or community.
- any research or institutional engagement involving human remains, funerary objects, sacred objects, or objects of cultural patrimony that are subject to the Native American Graves Protection and Repatriation Act.
If the answer is 'Yes,' then you must also enter the full name of any tribe(s) that will be involved.
- Federally recognized Tribal Nations should be selected/added from the drop-down provided. You may add additional federally recognized Tribal Nations by clicking the + box below. You may remove added federally recognized Tribal Nations by clicking the - box to the right of the Tribal Nation.
- Other Tribal Nations and indigenous communities (including those external to the United States) should be entered in the text box provided. Please be aware that this information is used for reporting, so your best efforts at providing useful, accurate information are appreciated. You can reach out to Claudia Nelson for assistance/guidance with what to enter in this text field.
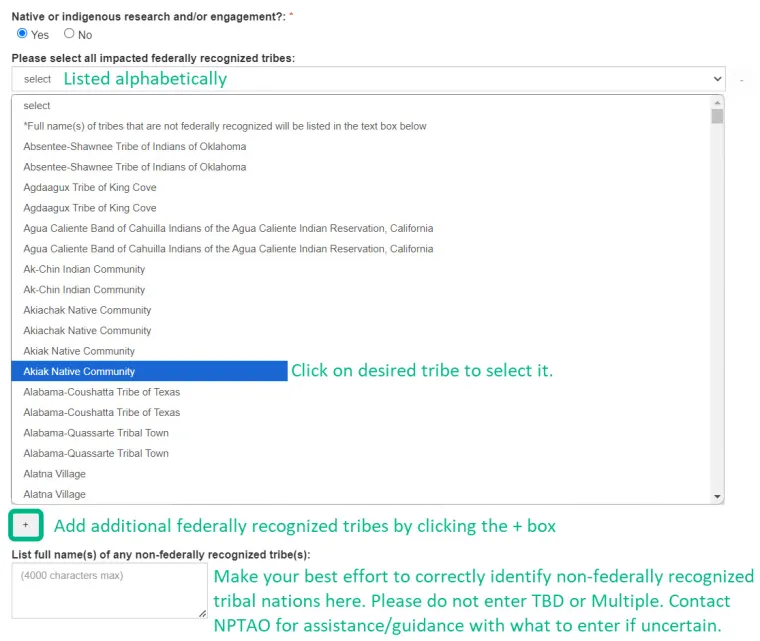
For questions or to learn more, please contact Claudia Nelson, Director, Native Peoples Technical Assistance Office (NPTAO) in Research, Innovation & Impact (RII).
For more information and resources, visit the Research & Engagement page on the Native American Advancement Initiatives & Research (NAAIR) website.
As a core service unit, Native Peoples Technical Assistance Office (NPTAO) serves as a primary research and resource liaison for Native affairs for the Office for Research, Innovation, and Impact (RII). This includes the Human Subjects Protection Program and Sponsored Projects review for compliance with ABOR 1-118 Tribal Consultation Policy and the UArizona Tribal Consultation Policy.
Enter the primary location where project activity will take place in the format Bldg-Rm-etc. Example: USB-510-A
The on- or off-campus designation of a project is determined by the location of the research and the wages incurred by the project. Separate On and Off Campus rates will not be used for a single project. The Off Campus Rate should be used for projects that are not conducted at university owned or leased facilities. Off Campus projects must include more than 50% of the wages incurred at a non-UArizona owned or leased facility. If lease costs are charged directly to the project budget, the project will be classified as Off-Campus.
Specific questions may be answered by Sponsored Projects Services (sponsor@arizona.edu(link sends e-mail)) in conjunction with Financial Services Rate Studies (ratestudies@fso.arizona.edu).
Radiation (Radioactive Material, Sealed Source, Radiation Generating Machine)?
Answer this question “Yes” if this proposal involves the use of Radiation.
Your work will need to be approved by the UA Radiation Safety Committee (RSC) before you can start work. You can access the documents needed to submit to the committee at https://research.arizona.edu/radiation-safety-forms, “Application for (Radioactive Material/Radiation Machine/Sealed Sources) Approval.”
Training is required before any person can handle Radioactive Material, Radioactive Sealed Sources or Radiation Generating Machines. Radiation Safety Training can be found on https://edgelearning.arizona.edu/. If you have any questions please reach out to rlss-rad-support@email.arizona.edu.
Non-Ionizing Radiation (Laser)?
Also answer this question “Yes” if this proposal involves the use of any laser or laser product.
Your work will need to be reviewed and approved by the UA Laser Safety Officer (LSO) and Laser Safety Committee (LSC). You can access the documents needed to submit to the committee at https://research.arizona.edu/compliance/RLSS/radiation-safety/laser-safety-program/laser-approval.
Training is required before any person can operate a laser. Laser training (Laser Radiation Protection Course (LRPC)) can be found on https://edgelearning.arizona.edu/. If you have any questions please reach out to rlss-rad-support@email.arizona.edu.
Answer this question ‘Yes’ if the proposal involves any of the terms below or involves any biohazardous and/or recombinant materials. For assistance, contact Research Laboratory & Safety Services at (520) 626-6850 or rlss-bio-support@email.arizona.edu(link sends e-mail) or visit the Recombinant and Biohazardous Materials web page.
If the project you are proposing involves the handling or storage of Risk Group 3 biohazardous and/or recombinant materials that would require the use of a BSL-3 laboratory, please contact Research Laboratory & Safety Services at rlss-help@arizona.edu. BSL-3 work must be approved in advance by the Institutional Biosafety Committee (IBC). You can access the documents needed to submit to the committee at Institutional Biosafety Committee | UArizona Research, Innovation & Impact. Access and training for the BSL-3 spaces is a lengthy process, so reaching out to RLSS early in the process is optimal.
- Adenovirus
- Adenoassociated Virus
- AAV
- Cloning
- Gene therapy
- Genomic library
- Microarray
- Plasmid
- Probes
- Recombinant
- Retrovirus
- Restriction fragment polymorphism (RFLP)
- Sequencing
- Southern / Northern hybridization
- Transformed cells
- Vector
- Bordetella pertussis
- Campylobacter
- Cell Culture
- Coccidiodes
- Chlamydia
- Clostridium
- Coxiella
- Cryptosporidium
- Cyclospora
- Giardia
- herpes
- HIV
- Influenza
- Monkeypox
- Mycobacterium
- Yesinia pestis (plague)
- Salmonella
- Toxin
- Vaccinia
- SARS-CoV-2
- Risk Group 2
- Risk Group 3
- Virus
- Bacteria
- Fungus
- Parasite
- Rickettsia
This data point will help campus units more accurately calculate proposal success or win rates. Units want to know what percentage of proposals that are reviewed and competitively scored against others for an open funding opportunity are ultimately funded.
Examples:
Answer 'Yes' if the proposed project...
- is an incoming subaward which is part of a proposal submission going in for review with the prime proposal at another institution
- will be reviewed and might or may not be awarded based on merit or a competition
Answer 'No' if the proposed project...
- if UArizona knows in advance that funding will be awarded, a funding decision has already been made and UArizona employs a certain person with unique expertise to accomplish project goals
- is a subaward from another institution that already received a prime award and UArizona is being asked to complete part of the work afterward
- will definitely be funded because UArizona has particular professional expertise
- is an administrative supplement that requires an application but funding is pre-approved
If you have any questions please contact sponsor@arizona.edu(link sends e-mail).
The Limited Submission opportunity will be listed in the Open category on the Limited Submission Table and the first expression of interest RDS receives will be accepted as the institutional submission. Expressions of interest must be sent to LimitedSubmissions@email.arizona.edu and the date/time that the email is received will determine the order that expressions of interest are accepted.
If proposal activity will take place at Banner University Medical Center (BUMC) facilities, answer 'Yes' to the appropriate location. These fields are not required so no response is the same as a 'No' answer.
- Banner - University Medical Center Tucson
- Banner - University Medical Center South
- Banner - University Medical Center Phoenix
All Investigators on each subaward will be required to complete the training and disclosure requirements. An Investigator means any person who shares the responsibility of Conducting Research. Conducting Research includes the design, development, testing, evaluation, conduct, reporting, review, and oversight of a program of scientific inquiry. Each Investigator will need to submit an individual disclosure.
In case helpful, below is a brief overview specific to subrecipients but more Information for Non-UA Subcontractors, Consultants, and Collaborators can be found on our website.
Subrecipients will be presented with two options. They will need to either (1) have an implemented and enforced federally-compliant conflict of interest policy and process and agree to be responsible for compliance under their policy and pursuant to the subcontract or consulting agreement including reporting requirements (our office reviews each subrecipient’s policy to determine it is compliant) or (2) comply with UArizona’s Conflicts of Interest & Commitment Policy (more details on the fees that apply can be accessed from link above). Our office will be in direct contact with the subrecipients to share a complete overview of all the related requirements. Our office will also provide any assistance and guidance the subrecipient may need.
Export control laws and regulations affect various University activities including, but not limited to conducting research (sponsored and unsponsored), international travel, publishing research, procurement, hiring non-U.S. persons, sponsoring foreign persons (e.g., visiting scholars), collaborations with non-U.S. individuals or entities, international shipments, non-disclosure agreements, and certain services to embargoed or sanctioned countries.
The UA provides both distributed and centralized assistance for proposal administration and development. When planning to develop and submit a proposal, please contact your departmental/unit business office for budget, routing, and submission. To enhance the competitiveness of your proposal, consider contacting RDS. RDS staff provide a range of (free) services from consultation regarding fit with solicitation to proposal management, including document review, technical editing, and more. RDS staff work hand-in-hand with you and your departmental/unit business office to ensure the complete and on-time submission of your proposal.
More information on college and departmental, as well as campus-wide assistance is described in the Institutional Capacity section of the Research Gateway.
Export controls are federal laws that govern the transmission of controlled items and associated technical data to foreign nationals. There are also federal regulations regarding providing services, traveling to, or working with individuals or entities from sanctioned or embargoed countries. These federal regulations not only affect items that are utilized by UA personnel, but can also affect whom the UA engages with on campus as well as around the world.
There are three primary agencies which govern export control laws and regulations: the U.S. Department of State Directorate of Defense Trade Controls; the U.S. Department of Commerce Bureau of Industry and Security; and the U.S. Department of Treasury Office of Foreign Assets Control.
Foreign affiliations are defined as associations/relationships (e.g. conducting activity such as consulting engagements, research collaborations, appointments or titles, or teaching) with foreign institutions of higher education, foreign governments, foreign companies or foreign nationals.
At the time of proposal submission to a federal funding agency, Investigators must have an up-to-date COI certification. That means each Investigator has:
1. Submitted either an Annual Disclosure Certification or a Research Certification in the last 364 days, and
2. Does not have any changes to an existing Outside Interest or new Outside Interest.
Investigators must:
1. Complete the Required COI Disclosure Training after July 1, 2021.
2. Complete the Required COI Disclosure Training once every 4 years thereafter.
Note: OROI may direct an Investigator to complete the training more frequently.
3. Submit an annual certification. This can be an Annual Disclosure Certification or Research Certification.
4. Update their certification within 30 days of a change to an existing Outside Interest.
5. Update their certification within 30 days of acquiring a new Outside Interest.
6. Submit a Research Certification for all non-sponsored and sponsored Research.
Research is not subject to export controls if it qualifies for at least one of three exclusions:
(1) Fundamental research exclusion;
(2) Public domain exclusion; and
(3) Education Information Exclusion.
Penalties for export control violations are substantial, including significant fines, debarment from participation in federal contracting, loss of export privileges, and in some cases imprisonment.
In addition to these severe penalties, the potential reputational damage to an institution from violation of these laws could be difficult to repair, possibly resulting in lost opportunities for attracting world-class researchers and/or decreased access to research funding.
UArizona will receive security updates and new system features for UAR on a continuous basis. The standard UAR system maintenance windows are:
- Daily from 7:00 PM - 8:00 PM MST
- Sundays from 6:00 AM - 10:00 AM MST
If you choose to work in UAR during the maintenance periods, please save your work often. While the system updates, you may have an interruption in service and unsaved work will be lost.
All surveys (both monthly and after-use) must include:
- Date survey was performed
- Initials of the surveyor
- A background count or count rate
- Location(s) surveyed
- Results for each location
- Follow-up survey results, if necessary
If the U.S. Government funds research and specific controls are agreed on to protect information resulting from the research, then information resulting from the project will not be considered fundamental research. Such controls are usually contained in contractual clauses. Examples of "specific controls" include requirements for prepublication “approval” by the Government; restrictions on dissemination of information to non-U.S. citizens or other categories of persons; or restrictions on participation of non-U.S. citizens or other categories of persons in the research.
- Perform a survey immediately .
- Inform the Approval Holder or Approval Safety Coordinator that a survey was missed.
A study can be deemed IND Exempt if all of the following criteria are met:
- If the study is not designed to support approval of a new indication or a change in label;
- If the study is not intended to support a significant change in the advertising for the product;
- If the study does not involve a route of administration, dosage level or patient population that significantly increases the risks (or decreases the acceptability of risk) associated with the use of the drug;
- The study is conducted in compliance with the IRB and Informed Consent regulations; and
- The study is conducted in compliance with regulations regarding promotion for investigational drugs.
The area must be immediately decontaminated and a documented follow-up survey must be performed to show that contamination does not exceed the action level.
Title case is used to provide consistency in the system and in reporting. Please do not use ALL CAPITALS or all lowercase in proposal/project titles.
In title case, major words are capitalized and most minor words are lowercase. If your title uses alternate capitalization where a letter other than the first letter of a word is used in the acronym and requires special emphasis, please capitalize as you feel is best indicated for your acronym.
Major words include nouns, verbs, adjectives, adverbs, pronouns, and all words of four letters or more.
Minor words include short (i.e., three letters or fewer) conjunctions, short prepositions, and all articles.
In title case, capitalize the following words in a title or heading:
- the first word of the title or heading, even if it is a minor word such as “The” or “A”
- the first word of a subtitle
- the first word after a colon, em dash, or end punctuation in a heading
- major words, including the second part of hyphenated major words (e.g., “Self-Report,” not “Self-report”)
- words of four letters or more (e.g., “With,” “Between,” “From”)
Use lowercase for minor words that are three letters or fewer in a title or heading (except the first word in a title or subtitle or the first word after a colon, em dash, or end punctuation in a heading):
- short conjunctions (e.g., “and,” “as,” “but,” “for,” “if,” “nor,” “or,” “so,” “yet”)
- articles (“a,” “an,” “the”)
- short prepositions (e.g., “as,” “at,” “by,” “for,” “in,” “of,” “off,” “on,” “per,” “to,” “up,” “via”)
Projects that meet the definition of regulated human research under the Common Rule may fit within one or more categories of “Exempt” research. This does not mean that these studies do not need IRB review. For a research study to be deemed "Exempt", investigators need to submit an application to the IRB. Please note that an "Exempt" determination must be made by the IRB.
Once a project is determined to be CUI it is managed under a security plan. The University of Arizona Export Control office worked closely with the IT-CUI team to develop “The Plan,” a joint Technology Control Plan and System Security Plan. This plan outlines the security measures researchers and staff must follow in order to protect the CUI data.
This action is an indication that the direction of the research or some other factor has changed the project in some way to render the export control regulations applicable to this project and that, more than likely, the researcher’s work will now be export controlled. Contact Export Control before continuing work on the project to re-evaluate for export control protocols.
If both the 7000 and 7012 clauses are in an agreement we can go back to the prime contracting officer and ask if the University of Arizona’s portion on the work is fundamental in nature. If we receive confirmation in writing from the prime contracting officer that the university’s work in fundamental it nullifies the CUI clauses.
The federal government is identifying emerging technologies essential to US national security and placing new or additional export controls on these technologies. Starting in 2019, the U.S. Commerce Department’s Bureau of Industry and Security (BIS) published export controls on several emerging technologies. Please review this summary of emerging technologies and let the Export Control team (export@arizona.edu) know if you have any research or projects in these areas so we can work with you to ensure compliance.
Human subjects training is required for all investigators, faculty, staff, and students conducting research with human subjects at the University of Arizona. There are two CITI training options for investigators to choose from, depending on the type of research:
- IRB: Biomedical Research Investigators (required for FDA-regulated research or projects involving accessing medical records); OR
- IRB: Social & Behavioral Research Investigators
Visit our Training Requirements webpage for more information.
Human Subjects Training Handout
HIPAA requires that a Covered Entity/Hybrid Covered Entity enter into a Business Associate Agreement (BAA) any time it will use a contractor or other non-workforce member to perform "Business Associate" services or activities on behalf of the Covered Entity. The purpose of the BAA is to protect the data and ensure that any party who performs functions/activities on behalf of the covered entity and will handle PHI in carrying out those duties adhere to certain standards to protect the data.
HIPAA requires that that a BAA must be written and must include several terms and conditions for maintaining compliance with federal privacy regulations, including written assurances that the Business Associate:
- Will not use/disclose PHI other than as permitted or required by the agreement or as otherwise required by law;
- Will use appropriate safeguards to prevent unauthorized use or disclosure of PHI (other than as provided for by the BAA);
- Will report any use or disclosure not provided for in the BAA for which it becomes aware; and
- Ensures that any subcontractors that create, receive, maintain or transmit PHI agree to the same restrictions/conditions as the business associate.
For more information about obtaining a BAA, contact the UA Privacy Office.
A Data Use Agreement (DUA) is a specific type of agreement that is required under the HIPAA Privacy Rule and must be entered into before there is any use or disclosure of a Limited Data Set (defined below) from a medical record to an outside institution or party for one of the three purposes: (1) research, (2) public health, or (3) health care operations purposes. A Limited Data Set is still Protected Health Information (PHI), and for that reason, HIPAA Covered Entities or Hybrid Covered Entities like The University of Arizona (UA) must enter into a DUA with any institution, organization or entity to whom UA discloses or transmits a Limited Data Set.
At a minimum, any DUA must contain provisions that address the following:
1. Establish the permitted uses and disclosures of the Limited Data Set--narrowly describes the use/disclosure and outlines parameters of specific purpose (research, public health or health care operations).
2. Identify who may use or receive the information;
3. Prohibit the recipient from using or further disclosing the information, except as permitted by the agreement or as otherwise permitted by law;
4. Require the recipient to use appropriate safeguards to prevent an unauthorized use or disclosure not contemplated by the agreement;
5. Require the recipient to report to UA any use or disclosure to which it becomes aware;
6. Require the recipients to ensure that any agents (including any subcontractors) to whom it discloses the information will agree to the same restrictions as provided in the agreement; and
7. Prohibit the recipient from identifying the information or contacting the individuals.
Additionally, Covered Entities, or Hybrid Covered Entities like UA, must take all reasonable steps to cure a recipient's breach of the DUA. For example, if UA learns that data it provided to a recipient is being used in a manner not authorized under the DUA, then notify the UA Privacy Officer and UA will work with the recipient to correct this problem. If these efforts are unsuccessful, UA would be required to cease any further disclosures of PHI to the recipient under the DUA and report the matter to the federal Department of Health and Human Services Office for Civil Rights.
A deemed export is the release or transmission in any form of export-controlled technology or software code within the U.S to anyone who is not a U.S. Person.
Restricted Party Screening (RPS) is the process of reviewing foreign and U.S. individuals and entities to prevent illegal transactions with parties on the various federal government lists of restricted individuals, companies, and organizations. or additional information see /compliance/export-control-program/procedures-for-restricted-party-screenings.
Financial Conflict of Interest (FCOI) means an Outside Interest is Related to, or can be perceived to be Related to, an individual’s institutional responsibilities.
FCOI determinations answer the question: Could it reasonably appear to someone outside of UA (e.g., front page of the newspaper) that a decision made in the conduct of research was influenced by your Outside Interest? That influence could affect the design of the project, a decision to exclude data, a decision to delay publication of research results, a decision to overemphasize or underemphasize research results, etc. for the benefit of your Outside Interest.
Related to is a defined term that refers to the condition in which it may reasonably appear that decisions made by the Investigator in the performance of his/her institutional responsibilities could directly and significantly affect the value of his/her Significant Financial Interests or be in conflict with Significant Personal Interests or Foreign Interests.
Relatedness includes situations in which an Investigator’s Outside Interests would reasonably appear to affect, or to be affected by, the individual’s Research or other institutional responsibilities.
Relatedness is not a judgment on whether an Investigator would deliberately make choices in the Conduct of Research or the performance of their Institutional Responsibilities based on considerations related to their Outside Interest. Rather, “Relatedness” refers to the condition in which it may reasonably appear that choices made in the Conduct of Research or other performance of the individual’s institutional responsibilities could be directly and significantly influenced by the existence of an Outside Interest.
A Limited Data Set is a data set that is stripped of certain direct identifiers specified in the HIPAA Privacy Rule. A Limited Data Set may be disclosed to an outside party without a patient’s authorization only if the purpose of the disclosure is for research, public health, or health care operations purposes and the person or entity receiving the information signs a data use agreement (DUA) with the covered entity or its business associate.
Limited data sets may include only the following identifiers:
- Dates, such as admission, discharge, service, and date of birth (DOB)
- City, state, and zip code (not street address)
- Age
- Any other unique code or identifier that is not listed as a direct identifier.
This means that in order for a data set to be a Limited Data Set, all of the following direct identifiers as they relate to the individual or his/her relatives, employers, or household members must be removed:
- Names
- Street addresses (other than town, city, state, and zip code)
- Telephone and fax numbers
- Email addresses
- Social security numbers
- Medical record numbers
- Health plan beneficiary numbers
- Account numbers
- Certificate/driver’s license numbers
- Vehicle identifiers and serial numbers, including license plate numbers
- Device identifiers and serial numbers
- URLs and IP addresses
- Biometric identifiers
- Full face photographic images and any comparable images.
NOTE: a Limited Data Set is still Protected Health Information (PHI) under HIPAA. It is not De-Identified Data, as that term is defined under HIPAA, and thus, must be safeguarded and protected as required under the Privacy Rule. For more information about the different between Fully Identifiable Data, a Limited Data Set and a De-Identified Data Set, check out the following HIPAA Data Reference Guide.
The FDA defines a medical device as:
- "an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar or related article, including a component part or accessory which is: recognized in the official National Formulary, or the United States Pharmacopoeia, or any supplement to them,
- intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease, in man or other animals, or
- intended to affect the structure or any function of the body of man or other animals, and which does not achieve its primary intended purposes through chemical action within or on the body of man or other animals and which is not dependent upon being metabolized for the achievement of any of its primary intended purposes."
Non-significant risk (NSR) devices are devices that do not pose a significant risk to the human subjects. Examples include most daily-wear contact lenses and lens solutions, ultrasonic dental scalers, and Foley catheters. An NSR device study requires only IRB approval prior to initiation of a clinical study.
The IRB is tasked with granting NSR determinations, when appropriate.
Sanctioned Countries are designated by the U.S. Government as having limited or comprehensive trade sanctions and embargoes imposed for reasons of anti-terrorism, non-proliferation, narcotics trafficking, or other reasons. Sanctions are prohibitions on transactions (e.g., financial exchanges, providing or receiving services of value) with designated countries, entities, or individuals.
A significant risk (SR) device presents a potential for serious risk to the health, safety, or welfare of a subject. Significant risk devices may include implants, devices that support or sustain human life, and devices that are substantially important in diagnosing, curing, mitigating or treating disease or in preventing impairment to human health. Examples include sutures, cardiac pacemakers, hydrocephalus shunts, and orthopedic implants.
When a study is investigating a SR device, an IDE from the FDA is required. IRB review will not occur until the study has been granted an IDE from the FDA.
The term includes drugs (including botanicals, biologicals, and gene therapy, and genetically derived products that meet the definition of a “drug”), and medical devices for human use. The FDA has statutory authority to regulate the development and marketing of these products.
Limited Submissions are funding opportunities where the funder has limited the number of applications from an organization. Research Development Services (RDS) facilitates Limited Submissions for federal and foundation opportunities for UA and ensures only eligible institutional proposals are submitted to the funder.
A “ticket” is the terminology RDS and Sponsored Projects & Contracting Services use to designate an approved institutional submission for a limited submission opportunity. The “ticket” will be sent to the approved PI/Team via email from Resdev@email.arizona.edu.
An Approval Holder (AH) is someone who is ultimately responsible for a laboratory or set of laboratories (administratively and operationally) that use or store hazardous chemicals. Usually, the AH is a Principal Investigator. The AH works with their Approval Safety Coordinator (ASC) and the RLSS to maintain compliance with the Laboratory Chemical Safety Program within his/her laboratories.
An ASC is a laboratory worker that is designated by the Approval Holder (AH) to undergo advanced chemical safety training, help with the responsibilities of the AH and to facilitate compliance with the Laboratory Chemical Safety Program. The ASC also has delegated authority from the AH to be a main point of contact for the RLSS.
The Assistance Listing Number (ALN), formerly known as the Catalog of Federal Domestic Assistance (CFDA) Number, is a five-digit number assigned in the award document for all federal assistance award mechanisms, including federal grants and cooperative agreements. It is used for governmental reporting and auditing.
The first two digits of the Assistance Listing Number reflect the federal grantor agency followed by a decimal point. The final three digits following the decimal) indicate the federal program funding the project within the agency. The partial list below represents some common funding agencies for UArizona. Assistance listing format examples: ##.###, 93.866
10: U.S Department of Agriculture (USDA)
12: U.S. Department of Defense (DOD)
15: U.S. Department of the Interior (DOI)
43: National Aeronautics & Space Administration (NASA)
45: National Endowment for the Arts & Humanities (NEA, NEH)
47: National Science Foundation (NSF)
64: Department of Veterans Affairs (VA)
81: U.S. Department of Energy (DOE)
84: U.S. Department of Education
93: U.S. Department of Health & Human Services (DHHS)
The US Federal Government renamed the "Catalog of Federal Domestic Assistance" (CFDA) to "Assistance Listing Number" (ALN). Agency websites and awards may transition to the new labeling. In June, 2023 this label change appeared in the UAccess Research (UAR) system, most notably in Proposal Development, Institutional Proposal, and Award. UAccess Financials continues to use the "CFDA" label. No matter which label is used, it is the same identifier!
An export is the transfer of export-controlled data, items, equipment, materials, and software or providing a defense service to a non-U.S. Person or entity. An export can occur in a number of ways, such as; a physical shipment, hand-carrying an item out of the U.S., email transmission of data, presentations, discussions, or visually accessing export-controlled data.
An investigational device exemption (IDE) is an approval that allows a medical device to be used in a clinical research study that involves human subjects or human specimens. The term “exemption” as it pertains to IDEs, means that the device is exempt from the laws that prohibit unapproved products to move in interstate commerce.
An approved IDE means that the IRB (and FDA for SR devices) has approved the sponsor’s study application and all regulatory requirements are met.
IDEs cover studies that:
- Support marking applications;
- Collect safety and/or efficacy information about a device;
- Evaluate device modifications;
- Collect device specific data about an unapproved device (even if there is no plan to submit a marketing application); or
- Support a new use of a marketed device.
An investigational drug is defined as:
- An article that is not approved (for marketing) in the US as a drug.
- An approved drug that is not used according to the approved label (or a new combination of approved drugs).
NIH describes Budgetary Overlap as: "Budgetary Overlap occurs when duplicate or equivalent budgetary items (e.g., equipment, salary) are requested in an application but are already provided by another source."
Executive Order 13556 “Controlled Unclassified Information,” (the Order), issued on November 4, 2010, established the CUI program, which standardizes and simplifies the way the Executive branch handles unclassified information that requires safeguarding or dissemination controls, pursuant to and consistent with applicable law, regulations, and government-wide policies. The National Archives and Records Administration (NARA) serves as the Executive Agent to implement this order and oversee agency actions to ensure compliance.
Drug labeling refers to all the printed material that accompanies a drug, including the label, the wrapping, and the package insert.
Laboratory-Specific Chemical Safety Training is required by the UA Laboratory Chemical Safety Program for all hazardous chemical laboratory workers, in addition to the online General Laboratory Chemical Safety Training. This training must be provided by the Approval Holder or Approval Safety Coordinator of a laboratory to every hazardous chemical worker under their Approval. The laboratory-specific training must cover, at a minimum, the storage and use of hazardous chemicals, required control measures when using hazardous chemicals, the location of safety equipment in the laboratory, and any other laboratory-specific plans, procedures or related hazardous chemical guidelines.
A template for this training can be found on the RLSS website. By affirming to reading and understanding the Laboratory Chemical Hygiene Plan, a laboratory worker also affirms to having been provided adequate Laboratory-Specific Chemical Safety Training. This fulfills the documentation requirements of the OSHA Hazard Communication Standard. The AH/ASC should maintain documentation of the information covered during the Laboratory-Specific Chemical Safety Training.
- Is published or disseminated in the Public Domain
- Arises during, or results from, fundamental research
- Is educational information released by instruction in catalog courses or associated teaching laboratories of academic institutions.
“Off-label use” is any difference in use, including indication, dose, route of administration, patient populations, and drug formulation from what is approved on the FDA label.
“On-label use” means the drug is being used in the same indication, dose, route of administration, patient populations, and drug formulation. There is no deviation from the approved FDA label. Studies involving the on-label use drug do not require an IND, as long as data will not be used in a marketing application.
NIH describes Scientific Overlap as: "Scientific Overlap occurs when substantially similar research is proposed in more than one application or is submitted to two or more different funding sources for review and funding consideration; or a specific research objective and the experimental design for accomplishing that objective are the same or closely related in two or more applications or awards, regardless of funding source."
“Significant Use of University Resources” includes but is not limited to: use of research funding; use of funding allocated for asynchronous or distance learning programs; use of telecommunication and data services beyond ordinary use; use of university computing resources; use of instructional design or media production services; access to and use of research equipment and facilities or production facilities; use of University Assets* such as paid employee time, proprietary information, intellectual property (such as patents, trademarks, and copyrights), logos, land and buildings.
The use of library resources, personal workstations, or personal computers are not typically construed as Significant Use of Board or University Resources.
*University Assets is fully defined in the University's Misuse of Assets Policy.
This clause requires the university to implement security measures as outlined in the NIST 800-171. In the event of a cybersecurity incident, the university’s responsibility under DFARS 252.204-7012 is to report the incident to the DoD within 72 hours. The university should preserve and protect images of all known affected information systems identified in this clause and all relevant monitoring/packet capture data for at least 90 days from the submission of the cyber incident report.
Disclosure of Information restricts the release of information unless the information is already in the public domain, the Prime Contracting Officer has given prior written approval, or the results during the performance of the project involved no covered defense information and has been determined by the Prime Contracting Officer to be fundamental research.
The after-use survey is performed to ensure your radioactive material (RAM) workspace is free of radioactive contamination after each use radioactive material. The monthly survey is performed to ensure all the rooms on your approval are free of radioactive contamination.
Internal funding programs are funded by UA administrations, colleges, or departments while external, sometimes called extramural, funding comes from a federal, state, or local agency, Foundation, or another non-UA source.
An “Upcoming” limited submission is currently accepting pre-proposals through a campus-wide competition with an established internal deadline. An “Open” limited submission means that the internal deadline has passed without any pre-proposals submitted for review: thus, the first expression of interest that RDS receives will be accepted as the institutional submission. A “Completed” limited submission means that the external deadline has passed, and no further proposals are accepted by the funder. “Completed” limited submissions on the Limited Submission Table record the names of the UA institutional selections that received the ticket to submit to the opportunity.
The educational information exclusion covers commonly taught in courses listed in catalogues and associated teaching laboratories of academic institutions in the United States.
Export Control works closely with various Liaisons across campus. Export Control established a liaison toolkit (checklists, forms, and procedures to determine if export control concerns exist). Examples of “red flags” include publication restrictions, foreign person restrictions, and projects related to military and space. Liaisons enable the University to be proactive in identifying/resolving issues. If you are interested in becoming a liaison contact Export Control.
The Fundamental research exclusion is a broad-based general legal exclusion to protect technical information (but not tangible items) involved in research from being controlled by export controls. In other words, research qualifying as “fundamental research” is not subject to export controls.
- The EAR definition of Fundamental Research means research in science, engineering, or mathematics, the results of which ordinarily are published and shared broadly within the research community, and for which the researchers have not accepted restrictions for proprietary or national security reasons.
- The ITAR defines Fundamental Research as basic and applied research in science and engineering conducted at accredited U.S. institutions of higher education where the resulting information is ordinarily published and shared broadly within the scientific community. Such research can be distinguished from proprietary research and from industrial development, design, production, and product utilization, the results of which ordinarily are restricted for proprietary reasons or specific national security reasons.
- University research will not qualify as fundamental research if the university or researcher accepts any restrictions on the publication of the information resulting from the research, other than limited prepublication reviews by research sponsors to prevent inadvertent divulging of proprietary information provided to the researcher by sponsor or to ensure that publication will not compromise patent rights of the sponsor.
NIST 800-171 Rev. 2: The National Institute of Standards and Technology Special Publication 800-171 provides agencies with recommended security requirements for protecting the confidentiality of Controlled Unclassified Information (CUI) when resident in Non-Federal Information Systems and Organizations. There are over one hundred security requirements in the NIST; this document is summary in nature and not an exhaustive list. See the NIST for complete details.
Primary Investigators (PIs) are responsible for:
- Assisting Export Control in the identification of activities that may intersect with export control regulations;
- Maintaining a current export control training certification;
- Confirming with Export Control all project personnel have completed training and are cleared to access export-controlled items;
- Notifying Export Control of potential violations.
Sponsored proposals are submitted for review and approval through the UA’s electronic research administration system, UAccess Research. Work with your departmental/unit business office for routing and submission of the proposal to Sponsored Projects & Contracting Services (SP&CS). SP&CS will submit the proposal on behalf of the institution. For more information visit the Proposal Submission section of the Research Gateway.
The public domain exclusion applies to information that is published and that is generally accessible or available to the public through:
- sales at newsstands and bookstores;
- subscriptions which are available without restriction to any individual who desires to obtain or purchase the published information;
- libraries open to the public or from which the public can obtain documents;
- patents available at any patent office;
- unlimited distribution at a conference, meeting, seminar, trade show or exhibition, generally accessible to the public, in the United States;
- public release (i.e., unlimited distribution) in any form (e.g., not necessarily in published form) after approval by the cognizant U.S. government department or agency.
Monthly surveys are required to ensure your approved radioactive material use/storage areas are free of radioactive contamination.
Research Development Grants are not designed as part of faculty retention or other reward but rather as a pathway to success for interdisciplinary research to accelerate competitiveness for extramural proposal submissions.
The RLSS User Dashboard is the major communication hub between the laboratory and the RLSS. It maintains pertinent records related to safety and compliance for the Approval Holder’s laboratory. These records include training requirements and completion records, laboratory hazardous chemical inventories, relevant plans (i.e. the University Chemical Hygiene Plan and the Approval Holder’s Laboratory Chemical Hygiene Plan), and worker affirmations to these plans. All laboratory workers under a Hazardous Chemical Approval have access to their training and affirmation records, can read and affirm to updated chemical hygiene plans, and access/edit that Approval’s hazardous chemical inventory and safety data sheet management system.
Arizona’s Technology and Research Initiative Fund investment has enabled thousands of scientific discoveries, over 950 patents, 328 new startup companies and hands-on training for approximately 39,000 students across Arizona’s three universities. Publicly supported through voter approval, TRIF is an essential resource for growing Arizona’s economy and providing opportunities for Arizona residents to work, learn and thrive.
Relevant reports can be found on the Arizona Board of Regents site.
U of A TRIF achievements are featured here.
University Administrators must disclose:
A University Administrator is any University Employee in a position of administrative leadership of a college, academic department, business, or other administrative unit, where a regular job requirement is to make institutional decisions on behalf of the University of Arizona. The role of a University Administrator includes, but is not limited to, the following positions (whether such positions are staffed on an interim, full-time, or part-time basis):
- University Senior/Associate/Assistant Vice Presidents
- University Provost
- University Senior/Associate/Assistant Vice Provosts
- University Deans
- University Vice/Deputy/Associate/Assistant Deans
- University Directors
- University Department Heads/Chairs
- University Business Officers/Managers
- University Division Chiefs, Center Heads/Directors
- University Employees with the authority to sign agreements on behalf of the University of Arizona or Arizona Board of Regents
- University Employees whose duties and responsibilities include contracting or services related to Research administration, Research contracting, Research compliance, responsible conduct of Research, sponsored projects services, or technology transfer and who are in a position to influence decisions or commit University resources in the performance of the University Employee’s duties and responsibilities.
- Individuals who serve as Chairs on the University’s Institutional Review Board committees, regardless of whether such individuals are University Employees.
RDS offers several programs on an annual or biannual basis.
- NSF CAREER Grant Preparation Program
- NIH R Series Grant Preparation Program
- Arts and Humanities Fellowship Preparation Program
Visit the Seminars and Workshops section to learn more about these programs and other training opportunities.
Every room listed on your RAM approval must be surveyed. Monthly surveys may be limited to RAM storage areas during calendar months in which RAM is only in storage and not in use.
- All areas where radioactive material is used or stored
- Commonly touched items, such as light switches, RAM storage area exterior surfaces, telephones, tools, equipment controls, door handles, or computer keyboards/mice
- Commonly travelled floor areas such as entrances or exits and the floor near RAM use areas, waste storage locations and frequently used equipment
The method depends on what radionuclide(s) your approval is approved to possess and use:
- Approvals approved for low energy beta emitters (such as C-14, S-35 and H-3 ) must use a liquid scintillation counter.
- Approvals approved for high energy beta emitters (such as P-32) can use a pancake probe Geiger counter.
- Approvals approved for low energy gammas emitters (such as I-125 or Cr-51) can use a low energy gamma scintillation probe.
- Wipes surveys with a liquid scintillation counter can substitute for portable instrument surveys.
Hazardous chemical workers must complete the online General Laboratory Chemical Safety Training provided through the RLSS online training website and receive laboratory specific training from their Approval Safety Coordinator or their Approval Holder. Additional training may be required, such as respirator or fire extinguisher training. Chemical Safety Training Information.
- Publication, access, and dissemination restrictions in the sponsored research agreement
- Foreign party restrictions stated in the sponsored agreement
- International travel to countries subject to U.S. embargoes and sanctions
- Sponsor is providing export-controlled technology, technical data, or equipment
- Non-U.S. students or visiting scholars participating in a restricted project
- Project is sponsored by the federal government or defense contractor
- Project is military, space-related, or has other implications to national security
- Project will be conducted abroad or with a foreign sponsor or collaborator
- Sponsor /entity/research/collaborator is in Cuba, Iran, North Korea, Sudan, or Syria
- Any shipment of goods, services, information, or technology abroad
If export controls are applicable, the project could require a TCP (Technology Control Plan) and/or an export license prior to commencement of activity. If you need an export control review please contact us. For sponsored projects, please complete the EC checklist.
We are working with Kuali to determine what we can do to improve these notifications to be clearer/more in line with a "Return for Edit". In the meantime, you can create a rule in Microsoft Outlook which marks the message as high importance and flags the message for follow-up that same day.
- Create Rule - In Outlook, Rules is in the top toolbar under the Move Section.
- Mark initial parameters and then select Advanced Options…
- Select any additional parameters or refine existing
- Select actions Mark it as importance and Flag message for follow up at this time. Then if you click on the hyperlinked text follow up at this time you can select Follow up Today and importance you can select high importance.
- Provide exceptions to refine or ensure you’re not applying the action to replied/forwarded items (when others are asking question) or copied items (where you’re not the main recipient).
Perhaps “except if the subject contains certain words” and include RE: and FW: as a filter criteria? Would help filter anything that was forwarded to them from others with a question.
- Name the rule and then check “Run this rule now” to run the actions against anything meeting those criteria currently in their box.
Monthly surveys are not required when there is no radioactive material in your inventory (including in storage and in waste) for the entire calendar month.
Or
The approval has been given a special condition from the Radiation Safety Committee(s).
Both the Proposal number and Document number were available and acceptable under UAR 5.2.1, however the Proposal number was more difficult to locate so the Document number became the preferred way to reference a proposal. In the updated UAR SaaS environment the Proposal number is more prominent and easier to locate, but SPS will still accept both numbers when referencing your proposal.
A DUA must be entered into before there is any use or disclosure of a Limited Data Set to an outside institution or party.
Monthly surveys must be performed every calendar month in which radioactive material is possessed.
There are a few potential explanations:
- You are searching for items you are not provisioned for. The Search Records feature only displays search results you have access to. You have access to items where you were the document initiator, you were included as personnel on the document, you were provided a specific viewer, editor, or aggregator role on the document, you are provisioned to view or approve documents for any of the included units, or you were added as an Ad Hoc with an Acknowledge, FYI, or Approve action. If you would like to see all search results, you will need to use the specific search functions for Awards, Negotiations, Institutional Proposals, Proposal Development, and Subawards in the Common Tasks section.
- Your search parameters are too narrow. You may need to broaden your search terms to capture additional items. The more search terms you combine (Lead Unit, PI, Title, Deadline Date, etc.), the narrower your results will be. By searching for broader categories, you will obtain more results.
- You are using an incorrect parameter for a given search term. You may need to look in the All Links section for the specific term to find out what possible parameters are and the specific spelling, spacing, and punctuation.
An IND is not needed for studies involving marketed drugs such as:
- Some studies using commercially marketed drugs
- Some studies using in vitro diagnostic biological products (e.g., blood grouping serum, reagent red blood cells, anti-human globulin)
- Studies using drugs in vitro or in laboratory research animals
When you enter into any commitment or obligation, even if it is not in writing, to Conduct Research for or on behalf of an individual or entity outside of UArizona (e.g., delivery of research results or data to the outside individual or entity) it constitutes an Outside Commitment and must be declared as such in advance of the activity, unless there is an existing signed agreement or contract between the outside individual or entity and the University of Arizona, on file with either RII Sponsored Project Services, RII Contracting Services or UAHS Research Administration, and which covers this specific commitment or obligation.
Non-sponsored research must be disclosed for conflict of interest review but is not an Outside Commitment that requires conflict of commitment approval unless you are Conducting the Research for or on behalf of an outside individual or entity.
Stock and equity valuation should be updated as follows:
Public Entity: Update at least annually, based on Annual Report or other public valuation
Non-Public Entity:
-
If original value is $4,999 or less, update within 30 days of value reaching $5,000 or more
-
If original value is $5,000 or more, update at least annually
See Also
RDS publishes the name of the PI selected for each Limited Submission opportunity on the Limited Submission Table on the Research Gateway.
Visit the Limited Submissions page on the UA Research Gateway to download guidelines and for a complete and searchable table of all upcoming, open, and completed Limited Submissions.
The RDS Limited Submissions Table page on the UA Research Gateway or Arizona Cultivate are regularly updated with all current funding opportunities, deadlines and updates.
1. When UA is disclosing or transmitting a Limited Data Set to another institution, organization or entity, UA requires that a DUA must be signed to ensure that the appropriate provisions are in place to protect the Limited Data Set as required under the HIPAA Privacy Rule. Contracting Services maintains a template DUA. When UA is disclosing or transmitting a Limited Data Set, if any material change is made to the UA template form, or if another party’s version of a Data Use Agreement is going to be used, Contracting Services must review and sign-off on the terms of the agreement. Email contracting@email.arizona.edu to request a DUA.
2. If a UA researcher is the recipient of a Limited Data Set from a non-UA source, the UA researcher will most likely be asked to sign the other party's DUA. In such instance, the UA researcher should consult with Contracting Services who will work to determine if it complies in material terms with UA’s DUA template. Email contracting@email.arizona.edu to request a DUA.
There are no specific requirements for where survey records must be maintained. Survey records must be readily available for inspection by Research Laboratory & Safety Services or outside regulatory agencies.
The applicant must first appoint a proxy from their account. The applicant will need to login to establish the account, and then indicate that a proxy will submit. Visit UA Arizona Cultivate for more details.
The RLSS is always available for assistance or guidance regarding laboratory chemical use by emailing rlss-chem-support@email.arizona.edu or calling 520-626-6850.
Additionally, other University departments are available for specific questions:
- Facilities Management: for facilities-related issues (e.g. fume hoods, fire extinguishers etc.) call 520-621-3000
- Risk Management Services: for waste disposal, air quality concerns, or respirator fit testing call 520-621-1790
For questions that require immediate attention, please email the general inbox at VPR-IRB@arizona.edu. This inbox is continuously monitored, and your questions will be directed to the appropriate individual.
Any radiation worker listed on your approval may perform and document a monthly survey.
A Business Associate is a person or entity who, on behalf of a HIPAA Covered Entity, or Hybrid Covered Entity like UA, performs or assists in the performance of a function or activity or provides support services, while not a member of the workforce, to the Covered Entity involving the use or disclosure of individually identifiable health information.
Some Business Associate functions or activities that may be performed on behalf of a Covered Entity/Hybrid Covered Entity include:
-
-
data processing
-
data analysis
-
utilization review
-
billing
-
cloud storage vendor services
-
transcription services
-
legal services
-
data aggregation
-
administrative functions
-
financial services
-
management services
-
consulting services
-
accounting services
-
legal services
-
actuarial services
-
accreditation services
-
An individual or organization is only considered a Business Associate if they perform a function or service on behalf of the Covered Entity/Hybrid Covered Entity (such as UA) and handle or are expected to Protected Health Information (PHI) as a part of the job function or service they perform/provide on behalf of the Covered Entity/Hybrid Covered Entity.
In some cases, UA may serve as a Business Associate of another Covered Entity if UA is performing services, functions or activities on behalf of the other Covered Entity and handling PHI as part of the services performed. When UA is acting in its capacity as a Business Associate and will be disclosing any of the Covered Entity’s PHI to a third party, a Subcontractor, to perform any of its services—UA is required to enter into Business Associate Agreement with any downstream Subcontractor that will have access to the Covered Entity’s PHI.
An Investigator is any person who shares the responsibility of Conducting Research.
This includes, but is not limited to, the Principal Investigator (PI), Co-PI, Co-Investigator, Project Director (PD), Co-PD, Senior/Key Personnel, and any other person, regardless of title or position, who is responsible for Conducting Research performed by or under the auspices of the University.
This does not, however, include individuals whose performance is purely ancillary. For example, office staff who provide ancillary support or hospital staff who provide intermittent care and do not make contributions to the research data are not Investigators.
Principal Investigators are responsible for identifying the Investigators who are participating in their research and the Office for Responsible Outside Interests will help Principal Investigators identify such individuals. Principal Investigators should consider the following:
- The significance of the tasks assigned to the individual with regard to Conducting Research (i.e., making a significant contribution to the research results by participating in the design, development, testing, evaluation, conduct, reporting, review or oversight of the research, or in all of these activities);
- The degree of independence the individual may have in performing their assigned tasks;
- Whether the individual will be directly involved in the research intervention or consenting or evaluation of human research subjects;
- Will the student/trainee be responsible for Conducting Research without direct oversight from an Investigator; and
- Whether the individual will be a collaborator or given authorship credit on a publication related to the Research or present Research findings at a meeting or conference.
For assistance in making this determination, you can review the following resources: Identifying Investigators & COI Disclosers and Who is an Investigator?, or contact OROI.
UA’s Contract Offices and Business Owners are responsible for:
- Determining if PHI is being shared with another entity, and if so;
- Determining whether:
- (a) UA is sharing its PHI (or the PHI UA holds on behalf of another Covered Entity in its capacity as a Business Associate) with a third party (company, sponsor, institution) and the third party is the Business Associate.
- (b) The third party (company, sponsor, institution) is sharing its PHI and UA is the Business Associate
Submit the Business Associate Intake Form to the UA HIPAA Privacy Office when a Business Associate Agreement is needed.
UA must enter into a Data Use Agreement (DUA) whenever it is transmitting or receiving a Limited Data Set, a type of Protected Health Information (PHI), for research, public health activities or health care operations.
UA Contract Offices and Principal Investigators (PIs)/Business Owners are responsible for:
1. Determining if a Limited Data Set is involved for a specific purpose (research, public health activities, health care operations), and if so;
2. Determining whether:
(a) UA is TRANSMITTING/DISCLOSING a Limited Data Set to a third party (company, sponsor, institution).
(b) UA is RECEIVING a Limited Data Set from a third party (company, sponsor, instiuttion).
3. Submitting a request to the Contracting Services email address contracting@email.arizona.edu when a Data Use Agreement is needed.
Principal Investigator (PI) eligibility is described in the Getting Started section of the Research Gateway.
Many large and/or complex proposals require significant preparation time for development and coordination. To provide the submitting PI or team the maximum amount of time to focus on the external application, internal Limited Submission contests are run well in advance of the external deadline.
While every effort is made to adhere to our preferred Limited Submissions timeline, there are instances when the interval between becoming aware of a limited submission and the sponsor’s deadline makes it impractical to follow our process. In those cases, broad announcements may not be made, expedited reviews with limited or no feedback comments may be performed, and/or submission slots may be awarded on a first-identified basis. Generally, if there are less than six weeks between the identification of the opportunity and the sponsor’s deadline, a competition will not be run, and the competition will be listed as Open or submission permission will be awarded on a first-identified basis.
The pre-proposal requirements are designed to give a sufficient representation of a prospective project or proposal without over-burdening faculty/applicants. Think of the pre-proposal as an “elevator pitch.”
The proposals that are populated in your disclosure are pulled from UAccess Research. If a proposal was not funded and it is showing up in your disclosure it is most likely because Sponsored Projects was not notified that the proposal was not funded. Please use the “Set as not required” option in your disclosure and select the appropriate reason from the drop down menu. Also, you or your business office should contact Sponsored Projects & Contracting Services to notify them that the proposal was not funded. They will set the proposal to the appropriate status.
As a public University and recipient of federal research funding, UArizona is required to comply with federal regulations, state law and ABOR policies. Additionally, UArizona has a fiduciary responsibility to ensure inappropriate external influences do not affect the performance of one’s primary duties to UArizona.
The information collected in eDisclosure allows UArizona to ensure development and implementation of management strategies in order to facilitate our faculty’s continued cutting-edge research and that all University Employees meet regulatory requirements.
More information on disclosure requirements can be found on our History of Disclosure Requirements webpage.
UArizona’s policy can be found here: Conflicts of Interest & Commitment Policy
To be compliant with OSHA regulations, all laboratory workers under a Hazardous Chemical Approval must read both the University Chemical Hygiene Plan and the Laboratory Chemical Hygiene Plan for that Approval. All laboratory workers must also affirm that they understand the information within these plans, and are aware that they can seek assistance from the RLSS and their Approval Holder if needed. Workers will be reminded by the RLSS User Dashboard to complete this affirmation annually, or upon amendment of the plans.
Using the RLSS User Dashboard, laboratory workers can easily access the University Chemical Hygiene Plan, as well as the Laboratory Chemical Hygiene Plans for any Approvals under which the laboratory worker is registered. Workers can then read and affirm to these documents through their User Dashboard after logging in with their NetID.
The Human Subjects Protection Program requires protocol personnel submit a Research Certification via eDisclosure for each project they are listed on in eIRB. For sponsored research, the Human Subjects Protection program has mapped IRB protocol roles to the sponsored project personnel categories. This mapping tool can be used to ensure protocol personnel complete Research Certifications: Investigator Mapping.
There is an overnight process that runs behind the scenes in UAccess Research (UAR) to make sure dashboard cards like “Proposals not routing,” “Proposals routing to me” and “Proposal Workload Assignments” are updated with the latest changes. This process usually begins a little after midnight AZ time. Proposals approved after midnight may not be reflected in the UAR dashboard cards that day, they will update with the next overnight update.
If a looming deadline cannot wait for the next overnight update, please email Sponsored Projects Services (SPS) at UARhelp@list.arizona.edu to request assistance. Include the Proposal number or UAR document number in the request.
The Search Records feature will return results excluding disapproved and cancelled documents, while the individual searches in Common Tasks (Awards, Negotiations, Proposals, Subawards) will return all results, including disapproved and cancelled documents.
Additionally, the Search Records feature is limited by your access/role, whereas the individual searches are not. Search Records will only return results for items you have access to, either because you created the document, you were provided an access role to the document, you are listed as personnel on the document, or you have been provisioned as a unit viewer/approver. You should be able to open any document that appears in your Search Records results. Individual searches in Common Tasks (Awards, Negotiations, Proposals, Subawards) will return all results, regardless of access, however you will only be able to open items for which you have access.
A recall or return for edit action removes any pending workflow Approval and Ad Hoc FYI/Acknowledge/Approve actions and basically resets routing.
If you are using an Ad Hoc FYI or Acknowledge to track a proposal in your Action List once it leaves your "Proposals not routing" card, you can take the Ad Hoc action and it will transfer to your Action List Outbox, where it will remain even if the proposal is recalled or returned for edit.
To turn on our Action List Outbox:
- From your Action List, click the preferences button in the upper right corner.
- Ensure Use Outbox is checked in your Fields Displayed in Action List.
- Be sure to click the save button at the bottom of the page!
At times your Action List Outbox may get very large and may take more time to load and process pages. You can delete older items if and as desired to pare down your Outbox and make it run more efficiently.
To delete old items from your Action List Outbox:
- From your Action List, click Outbox.
- Check each item you wish to delete.
- Click delete selected items.
No. RDS does not publicly identify the members of the internal funding review panels or the specific members assigned to individual proposals or ad hoc reviewers.
Yes. Review comments are provided to all applicants once the reviews are completed, and the ticket has been awarded.
Yes. Your Automatic Refresh Rate, Action List Page Size, Fields Displayed, Color Coding for Route Status, and Email Notification Preferences will all be migrated over to the updated UAccess Research system.
No. Campus users may only create Proposal Development documents. Only Sponsored Projects, the Office of Research Contracts, and UAHS Contracts will be able to create Institutional Proposals, Negotiations, Awards and Subawards. You will receive an error message if you attempt to create any of these document types and do not have the appropriate provisioning.
You will have access to items in the 'Proposals not routing' card on the dashboard if you:
- initiated the proposal document
- are included as Personnel on the project (PI, Co-I, Key Person)
- are provided viewer, editor, or aggregator privileges through the Access tab of the proposal
- are provisioned to view or approve proposals for any unit included in the proposal
- are added as an Ad Hoc with an Acknowledge, FYI, or Approve action
If a project involves work on a U.S. military base abroad, additional international travel insurance requirements may be required. For expert guidance, contact the UA Export Control Program well in advance of travel.
Defense Base Act (DBA) Insurance is a federal requirement for international travel that is associated with the U.S. federal government. There are two primary triggers when DBA must be obtained:
- Travel abroad as part of a public work or service contract with the U.S. federal government where the UA is a contractor or subcontractor
- Travel to conduct work on a U.S. military installation abroad
DBA is generally NOT required for travel under federal research grants, cooperative agreements with federal agencies, or other authorized university travel unless one of the two triggering criteria listed above are applicable.
To arrange DBA insurance, ask your departmental Business Officer to complete a DBA Insurance Application Form and submit it to Risk Management Services a minimum of 30 days in advance of departure. Email confirmation of DBA coverage will be sent to the traveler and their department. Contact Risk Management Services at (520) 621-1790 or risk@email.arizona.edu for additional forms information.

